I am so grateful that I got Evusheld on Wednesday. There were concerns I might react to it, primarily because I also have Mast Cell Activation Syndrome, but it went fine. I feel fortunate to have access to it.
COVID Vaccines, Evusheld & Stopping Drugs for Shots [FEB 2023 Update]
Stopping Methotrexate for 1 Week as Effective as 2 Weeks after Flu shots [FEB 2023 Update)
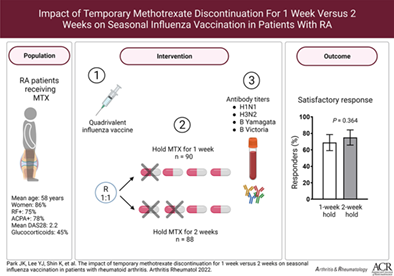
A February 2023 study shows that stopping methotrexate for just 1 week after the flu shot in rheumatoid arthritis (RA) patients was “noninferior” to holding methotrexate for 2 weeks. (See the study design in the image above). This means that almost as many patients had a good response to the flu shot after just missing one dose of methotrexate compared to missing 2 doses after their shot. This is important because we know that methotrexate decreases the response to flu shots and stopping methotrexate afterward can increase response rates. However, the longer you stop someone’s methotrexate, the higher their chances of having a flare. This will most likely significantly impact clinical care.
I’ll now have my patients stop methotrexate for just one dose after their vaccines instead of two doses, which I had recommended in the past. They’ll most likely still respond better to the vaccine (by skipping one dose of methotrexate) but be at lower risk of flaring. Though this study was in RA patients, I’ll do the same in my lupus patients.
Evusheld no longer recommended by the CDC [JAN 2023 Update]
January 26, 2023, the CDC recommended not giving Evusheld any more. Only 10% of COVID cases in the US were due to the older strains that Evusheld protected against. Evusheld does not protect well against the newer Omicron variants which were 90% of cases. We were instructed to hold onto our Evusheld samples just in case the older strains become more common in the future
5th COVID Vaccine Recommended
The CDC now recommends getting a 5th COVID vaccine (if you have had either Pfizer or Moderna. This should be provided at least 4 months after your 4th vaccine.
If your first shot was the J & J, hopefully you’ve had 2nd and 3rd shots with Pfizer or Moderna. In this case, a 5th dose is now recommended 4 months after the 3rd dose.
Also, PLEASE consider getting Evusheld. It reduces COVID infections by a whopping 83% in a trial involving over 5000 participants over 6 months follow-up. We give it in our office, or click on the locator website below.

The research and knowledge about the prevention of COVID-19 infection complications are constantly in flux as research evolves. It is one of the most studied infections ever.
This blog post from Dr. Donald Thomas of The Lupus Encyclopedia covers:
- 5th COVID vaccines: Who should get them and why (4th vaccine if you got J&J)
- Stopping immunosuppressant drugs for COVID vaccines
- Evusheld: A new therapy preventing severe COVID-19 infection complications – who should get it?
Ask Your Doctor
Please always ask your doctor about your situation or any updates or changes to this information. Also, expert opinions may differ. These recommendations are based upon what doctors call “best medical evidence.” However, in some situations, the “best medical evidence” is weak, and that is where controversies can exist. For example, in the recommendations on what drugs to stop after COVID vaccines, the doctors on the COVID vaccine task force of The American College of Rheumatology (ACR) could not agree on stopping the anti-cytokines, such as tumor necrosis factor inhibitors (like Remicade and Humira). The only anti-cytokine inhibitor that was agreed upon to stop was belimumab (Benlysta); see below.

A 5th COVID vaccine? Who? Why?
The primary COVID vaccine series for the general population (normal immune systems) pertains to the 1st two vaccines in a series (such as two Moderna shots 4-8 weeks apart from each other, or two Pfizer shots 3-8 weeks apart from each other). The 8-week interval is recommended for males 12-39 years old to reduce the vaccine’s risk (rare) for heart inflammation (myocarditis).
However, people with compromised immune systems may not respond adequately to the “primary COVID vaccine series.” This includes those taking immunosuppressant drugs, as mentioned below, or people with immunodeficiency disorders. They should receive a 3rd “supplemental dose.” For people with immunocompromised systems, this 3rd supplemental does is considered part of the “primary series”.
For example, someone with lupus who is on mycophenolate or methotrexate may not respond well and should receive a 3-shot series rather than just a 2-shot series of shots. This 3rd shot is NOT called a “booster shot.”
The 4th Shot
When an immunosuppressed patient who has received a 3-shot COVID primary vaccine series is ready for their booster shot, it is a 4th shot. (In the general population, they receive a 2-shot series followed by a 3rd booster shot.)
4 months after the 4th shot, you should get a 5th Moderna or Pfizer vaccine.
In patients who have systemic lupus erythematosus, most who are only on hydroxychloroquine only need a total of 4 shots.
My patients who are also on an immunosuppressant (such as azathioprine, methotrexate, mycophenolate, belimumab, rituximab, voclosporin, etc.) should have a total of 5 COVID-19 shots. Suppose a patient is only on hydroxychloroquine and has an immunodeficiency disorder (such as hypogammaglobulinemia). In that case, that person may also need 5 shots instead of just 4 shots.
NOTE: if you have systemic lupus, make sure to read and follow The Lupus Secrets to do best with your lupus.

When should the 5th shot (booster) be given?
A 5th shot should be given 4 months (or later) after the 3rd (supplemental) mRNA vaccine in immunocompromised individuals.
How about if I already got the J&J vaccine?
It is not recommended for patients with a rheumatic disease (such as systemic lupus) to receive the J&J vaccine.
If someone is on an immunosuppressant drug (as listed below) or has an immunodeficiency disorder, received the J&J (Janssen) vaccine, and is not expected to respond well to the J&J vaccine, then a supplemental COVID vaccine shot should be given using an mRNA vaccine (Pfizer or Moderna). This shot should be given at least 28 days after the J&J shot.
The booster shot (3rd shot in total) for immunocompromised patients who initially received the J&J vaccine should also be a Pfizer or Moderna shot and should be given at least 2 months after the 2nd shot (mRNA vaccine) provided above.
They should then receive a 4th shot (Pfizer or Moderna) 4 months after the 3rd shot.

The Recommended COVID-19 Vaccine Schedules “in a nutshell.”
For immunocompromised patients (immunodeficiency disorders and people on immunosuppressants):
Pfizer patients:
- Shot #2 = 3-8 weeks after shot #1
- Shot #3 = 4 weeks after shot #2
- Shot #4 = (booster) 3 months after shot #3
- Shot #5 = (booster) 4 months after shot #4
Moderna patients:
- Shot #2 = 4-8 weeks after shot #1
- Shot #3 = 4 weeks after shot #2
- Shot #4 = (booster) 3 months after shot #3
- Shot #5 = (booster) 4 months after shot #4
J&J patients:
- Shot #2 = (Pfizer or Moderna) 4 weeks after shot #1
- Shot #3 = (Pfizer or Moderna) 2 months after shot #2
- Shot #4 = (booster) 4 months after shot #3

The Centers for Disease Control has approved Mix and Match for COVID-19 booster shots
What is a “Mix-and-Match COVID-19 Booster Shot?”
The CDC has approved something called “mix-and-match,” where someone can get a COVID vaccine that is different than their initial primary series. It is also referred to as a “heterologous booster dose.” The CDC recommends getting the same vaccine for the primary series (such as getting a Pfizer shot after your first Pfizer shot).
However, adults 18 years old and older may choose to get a different shot (preferably Moderna or Pfizer) for their booster shot. This is called a “mix-and-match” booster or heterologous booster dose.

Why should I continue to practice “physical distancing” after vaccination?
We recommend that patients with SLE (and others with abnormal immune systems) who have been vaccinated still practice strict social distancing.
Why? SLE and the medicines used to treat it (that suppress the immune system) can affect all parts of the immune system. Just think of the immune system as many different players. The vaccines primarily work on increasing the activity of the B-cells (a type of white blood cell) to make antibodies against the COVID-19 coronavirus. However, many other parts of the immune system (such as T-cells, macrophages, monocytes, neutrophils, dendritic cells, the spleen, etc.) can be affected by lupus and immunosuppressant drugs. You are still at increased risk of infection and complications of infection compared to the general population related to these other areas not working normally. However, unvaccinated SLE patients still do not have as much protection.
So, you need to do both. Continue practicing physical distancing, and get your vaccine.
Should I still get vaccinated even if I have already had COVID infection?
Yes. Being vaccinated after a prior infection provides stronger and longer-lasting immunity than does “natural immunity” from being infected by itself. You should wait until after your quarantine period before getting your vaccine.

What drugs to stop for COVID vaccines?
The response to the COVID-19 vaccines can be decreased by some drugs that suppress the immune system (called immunosuppressants). Reduced responses to COVID vaccines were 1st shown in organ transplant patients and then confirmed in other immunosuppressed patients using mRNA and other COVID vaccines in rheumatic disease patients.
Therefore, the American College of Rheumatology (ACR) recommends stopping some medications after COVID vaccines. However, patients at high risk of flaring off their medicine may not want to stop their medications. The risks and balance of trying to help the vaccines work better (by stopping drugs that suppress the immune system) while not causing the person’s systemic inflammatory disease (such as lupus, Sjogren’s, or RA) flare is important. Ask your doctor first.
Drugs that do not need stopping after COVID vaccines: chloroquine, hydroxychloroquine (Plaquenil), sulfasalazine
Drugs to stop for 1-2 weeks after COVID vaccines: abatacept SQ (Orencia SQ), azathioprine (Imuran), baricitinib (Olumiant), belimumab (Benlysta), cyclosporine, leflunomide (Arava), methotrexate (Otrexup, Rasuvo), mycophenolate mofetil (CellCept), mycophenolic sodium (Myfortic), tacrolimus, tofacitinib (Xeljanz), upadacitinib (Rinvoq), voclosporin (Lupkynis)
The ACR COVID vaccine task force committee was unable to reach a consensus on whether the following drugs should be stopped or not: anifrolumab (Saphnelo), adalimumab (Humira), anakinra (Kineret), certolizumab (Cimzia), etanercept (Enbrel), golimumab (Simponi), infliximab (Remicade), sarilumab (Kevzara), tocilizumab (Actemra), ustekinumab (Stelara)
Special Recommendations
- Abatacept IV (Orencia IV): Give the COVID vaccine 4 weeks after the previous IV dose of Orencia and give the next dose of IV Orencia one week after that. In other words, have a 5-week spread between Orencia infusions (instead of the usual 4 weeks) while giving the vaccine at the 4th-week mark.
- Rituximab (Rituxan): Give the COVID-19 vaccine 2-4 weeks before the rituximab infusion. Having as long a time as possible after the previous rituximab infusion is optimal. For example, let’s say that someone gets two infusions of rituximab every 6 months. If it is possible to push a round of rituximab further out (such as to the 7th or 8th month instead of 6th month) to allow more time after the previous rituximab treatment before giving the vaccine would be best, if it does not risk placing the patient at high risk of flaring from their systemic autoimmune disease. NOTE: All patients on rituximab should strongly consider Evusheld treatments as an extra precaution in preventing severe COVID-19 infection complications (see below).
- Cyclophosphamide: Give IV cyclophosphamide 1 week after a COVID-19 vaccine, if possible.then

Evusheld: treatment preventing COVID-19 infection complications
On 12/8/21, Evusheld (tixagevimab + cilgavimab) monoclonal antibody treatment was granted “emergency use authorization” (EUA) by the U.S. Food and Drug Administration (FDA) as prophylaxis (pre-exposure) to help decrease complications from COVID-19 infection. EUA designation was given for use in people 12 years and older who weigh at least 88 pounds (40 kilograms) and who are either immunocompromised (immunosuppressed) or who are unable to get a COVID-19 vaccine (such as those with severe reactions to a previous COVID-19 vaccine).
The production and distribution of Evusheld have been very slow. Each state and government facility are only getting a small number of doses. For example, as of writing this post, the state of Maryland has received only 5000 doses. The Walter Reed National Medical Center has not even received their doses, yet.
Therefore, it is only being offered in a small number of centers (such as hospitals and some pharmacies) and to patients at highest need. Most centers primarily give this treatment to organ transplant patients and patients treated with B-cell depleting therapies, like rituximab (Rituxan).
It is given as a shot of tixagevimab in one buttock, and another shot of cilgavimab in the other buttock, then repeated every 6 months after that.
If you recently had your COVID vaccine, you need to wait at least 2 weeks before getting your Evusheld.
Make sure to have your doctor fax in order ASAP so that you are higher up on the list (first come, first served). You can always refuse if you change your mind later.
Who can get it?
Although treatment is being restricted to the groups above (e.g., rituximab patients), others will most likely be able to get it once production increases. For example, in my area, one hospital is now also offering Evusheld to patients being treated with other biologics other than just rituximab.
Where can you get Evusheld?
You can locate sites on the map here: at the link. It is very easy to search for locations using your zipcode:
https://healthdata.gov/stories/s/COVID-19-Public-Therapeutic-Locator/chu2-wqes
Bottom Line: Everyone at high risk of doing badly from COVID-19 infection should consider getting Evusheld. However, it will be triaged or allocated to those at highest need first (initially Rituxan patients).
All of us must remain humble and open-minded as COVID-19 continues to baffle us and keeps us on our toes. Especially as science and research show us new and different things. If you are one of my patients, do not be surprised if I tell you something different one month from now.
An excellent summary of COVID vaccine recommendations as of February 2022 can be found here:
https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
Have you received your 4th or 5th COVID vaccine?
Have you been treated with Evusheld?
Please leave comments about your experiences below.
For more in-depth information on COVID Vaccines, Evusheld & Stopping Drugs for Shots [FEB 2023 Update]:
Read more in The Lupus Encyclopedia, edition 2
Look up your symptoms, conditions, and medications in the Index of The Lupus Encyclopedia
If you enjoy the information from The Lupus Encyclopedia, please click the “SUPPORT” button at the top of the page to learn how you can help.
What are your comments and opinions?
If you have lupus, what has your experience been? What do you recommend for other patients?
Do you have any questions to ask Dr. Thomas?
Please click on “Leave a Comment” above to comment.
Please support “The Lupus Encyclopedia” blog post page
Click on “SUPPORT” at the top of the page to learn how you can support “The Lupus Encyclopedia“
17 Comments

 Marlene Raper
Marlene RaperHi Dr. Thomas thank you for all you do to help us. I have SLE and I only take hydrixichloroquine. I received the first 2 Pfizer vaccines and also the booster. Was the booster the correct vaccine dose for me or should I have received the full dose for my 3rd vaccine. I’m not sure from the article above what it should be. Thank you so very much for your help.
 Donald ThomasModerator
Donald ThomasModeratorDear Marlene: Thanks for your kind words and question. The booster shot for people who received Pfizer and without moderate to severe immunocompromised states is getting a 2 part series of Pfizer shots (as you note) followed by a 3rd booster shot that is full dose.
The half dose is only for the Moderna booster. Donald Thomas, MD
 Robin T
Robin THello Dr. Thomas.
I received Moderna #1, #2 and #3. Did I receive a half dose of Moderna #3? I continue to take methotrexate and more recently added hydroxychloroquine. From what I read in your blog post, I should definitely have dose #4. Should it be Moderna again or Pfizer?
I refer to your book over and over again!!! Thank you for your knowledge and guidance. Donald ThomasModerator
Donald ThomasModeratorRobin: You’d have to ask whoever gave you shot #3. A 4th Moderna shot is the formal recommendation, to be done 3 months or later after your 3rd dose. Remember to stop MTX for 2 weeks after the shot per the ACR guidelines, but only if you are low risk of flaring and are in remission (ask your doctor first). Thanks for reading my book. I think you will really love the 2nd edition when it comes out (probably in one year)… better and improved with the help of over 50 additional lupus experts… Donald Thomas, MD
 Kate
KateMy pharmacy wouldn’t give me a 4th shot last month. They said Leflunomide is not a qualifying immunosuppressant. When I dove into the CDC website I eventually found why they said that. The definition of moderately to severely immunocompromised was tightened recently to exclude DMARDs like Methotrexate and Leflunomide often taken for Lupus.
The more detailed language for “Active treatment with high-dose corticosteroids or other drugs that may suppress their immune response.” is now “Active treatment with high-dose corticosteroids (i.e., 20 or more mg of prednisone or equivalent per day when administered for 2 or more weeks), alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, cancer chemotherapeutic agents classified as severely immunosuppressive, tumor necrosis factor (TNF) blockers, and other biologic agents that are immunosuppressive or immunomodulatory.”, excluding DMARDs.
Although the cdc website says you can self attest you’re immunocompromised, my pharmacy asked for details, and they have my med records of course, and refused me. I don’t want to try other pharmacies just to get denied, especially now that I know I don’t meet the criteria.
Thoughts? Mostly asking as I’ve seen multiple websites including this one saying that people on DMARDs like me qualify and should go get it, but per the cdc we don’t. I couldn’t find info on immunocompromised people recently getting denied for a 4th shot, only very early this year.
New short immunocompromised description: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html
New long immunocompromised description: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html
 Donald ThomasModerator
Donald ThomasModeratorSorry to hear that, Katie. That was incorrect of your pharmacy. A lot more goes into determining if someone is immunocompromised or not. Of course, now, a 4th shot is recommended for everyone 50 years and older, no matter what. I hope you got yours by now.
Early on, I also had patients like you get turned away. So, I just started giving a RX to my patients to take with them stating my patient is “immunocompromised.” The physician’s statement trumps a pharmacist’s “opinion” who does not really know the entire clinical scenario.
NOTE: This reply was corrected 6/6/22. a 2nd booster is indicated for healthy 50 and older adults but not healthy 10 to 49 yo adults… thanks, Kate!
Donald Thomas, MD
 Kate
KateWhere does it say a 4th shot is recommended for everyone 18+? I’m only seeing a CDC recommendation for 50+ and the new moderately to severely immunocompromised definition. Thank you.
 Donald ThomasModerator
Donald ThomasModeratorKate: Sorry about that, you are absolutely correct and that is a typo on my part… it should read 50 yo and older if not immunocompromised. I’ll edit my response and I ap[preciate your correcting me.
Dr T
 Marlene Raper
Marlene RaperThank you Dr. Thomas for all you do to help us. I have SLE and I only take hydroxichloroquine and 81mg asprin. I’m wondering if I Should get Evusheld?
 Donald ThomasModerator
Donald ThomasModeratorMarlene: thank you for your kind remarks.
There are other things that can cause immunosuppression that would lead me to have a HCQ monotherapy patient get Evusheld. Things such as advanced age, low gamma globulin, low white blood cell count, diabetes, history of radiation therapy, malnutrition, liver disease, kidney dysfunction, alcohol, if have a high risk of doing poorly if infected, if in a position where infection risk is high, and others. It is best to ask your rheumatologist. You can show this message if you like. Here is a nice link listing causes of immunodeficiency: https://www.merckmanuals.com/home/immune-disorders/immunodeficiency-disorders/overview-of-immunodeficiency-disorders
I wish you all the best.
Donald Thomas, MD
 Francis N Allen
Francis N AllenI have had no adverse reaction to the first Evusheld shot or my fourth Phiser shot. Will make an appointment for the fifth Phiser And second Evusheld shots
 Donald ThomasModerator
Donald ThomasModeratorThanks for adding the comment, Francis. Patients hearing from other patients can be reassuring and helpful.
Donald Thomas, MD
 shannon m jackson
shannon m jacksonThe news is not talking much about Evusheld; why is that.
 Donald ThomasModerator
Donald ThomasModeratorShannon, my suspicion is that it deals with such a small proportion of the population (moderately to severely immunosuppressed people). This is in contrast to vaccines and treatments for COVID, which affect almost everyone.
Donald Thomas, MD
 Sheri Rosen
Sheri RosenThe CDC is recommending RSV vaccinations for people over 65. What about people with lupus over 65? In about two weeks, I will start Benlysta infusions. My doctor thinks I should get RSV now, but I can’t find any evidence of the safety for people with autoimmunity. Can you? There seems to be a scare that older people are going to get exposed to a lot of RSV this fall.
 Donald Thomas MD
Donald Thomas MDSheri: I would recommend getting it sooner than later. A paper came out this week from John’s Hopkins showing that Benlysta decreases response rates to COVID vaccines (so I’d want my patients getting all vaccines as soon as they could prior to starting Benlysta. Infections are the 2nd most common causes of death in SLE and most , like RSV, are preventable. True…. It has not been studied in lupus patients. But so far, no vaccine has been shown to be generally dangerous to lupus patients…. Ie the potential benefits outweigh potential risks. But risks for any treatment is never zero (even too much water can kill someone)… so you need to go with what you feel most comfortable considering the current knowledge. I hope that helps your decision making…. Dr T




Leave a comment