I have been waiting for this drug to be approved! I suffer from terrible SLE and rashes with joint pain. I am talking to my rheumatologist this month about going on Saphnelo.
Anifrolumab FDA Approval to Treat Systemic Lupus [Saphnelo February 2025 Update]
Anifrolumab (Saphnelo) is the 2nd FDA-approved biologic to treat systemic lupus erythematosus (SLE). The first biologic was belimumab (Benlysta).
This article discusses Saphnelo in detail. To compare Saphnelo and Benlysta, see my other article on this topic: lupusencyclopedia.com/saphnelo-vs-benlysta
_______________________
NOTE: Johns Hopkins University Press, publisher of The Lupus Encyclopedia, is a nonprofit publisher. If you purchase JHUP books, like The Lupus Encyclopedia, you support projects like Project MUSE.
_______________________
Anifrolumab FDA Approval to Treat Systemic Lupus Erythematosus (SLE)
AstraZeneca announced its anifrolumab FDA approval on 8/2/2021. In this blog, learn how anifrolumab works, how it is given, and its potential side effects. If you are a patient with lupus that is not in remission, this brings you hope for a potentially better treatment, read more below. If you are a healthcare provider searching for detailed information on the indications, dosing, and precautions, read more below.
The anifrolumab brand name is Saphnelo
History was made when the U.S. Food and Drug Administration (FDA) gave final approval to use anifrolumab (Saphnelo) as a safe and effective treatment for SLE. Note that anifrolumab is the generic drug name, and Saphnelo is the trade (brand) name. It is produced by AstraZeneca.

How was history made with the anifrolumab FDA approval?
- The last time the FDA approved a drug for SLE was in March, 2011: belimumab (Benlysta). Before that, the only other FDA-approved drugs for lupus were aspirin in 1948, later steroids, and finally hydroxychloroquine in 1955. Just think, the last time a drug was FDA-approved to treat lupus, before belimumab, was at a time when color television sets were just popping up in U.S. households.
- Anifrolumab is the “first of its class.” It directly inhibits the activity of type 1 interferon, and no other drug does this.
- And more importantly, it provides HOPE to all those SLE patients who have failed so many other medications.
- In addition, the FDA approved its use as the 2nd biologic drug for the treatment of SLE.
How does anifrolumab (Saphnelo) work?
An example of how the immune system works normally
- First, it helps to understand some basics of how some parts of the immune system work. The way that the white blood cells of our immune systems talk to each other is by sending each other chemical messages called “cytokines.” Cyto– comes from the Greek meaning “cell,” and –kine comes from the Greek meaning “movement.” So, a cytokine is a molecule that causes cells to move.
Think about it. We need white blood cells to move properly to diseased areas of the body. We want them to move in large numbers, as fast as possible, to areas of our body where there may be foreign invaders (bacteria infection, virus infection, new cancer cells, a rose bush thorn in the skin, etc.).
For example, let’s say you have bacteria infecting the back of your throat, causing you to have strep throat. Some white blood cells (such as plasma dendritic cells) recognize these as foreign invaders and secrete cytokines (molecular signals) into the bloodstream and lymph channels. These cytokines tell other white blood cells in the blood and lymph nodes that they need to go ASAP to the throat to kill the bacteria. These white blood cells do the same thing. They also secrete cytokines telling even more white blood to go to the infected throat area.
White Blood Cells and Inflammation
Unfortunately, these white blood cells at the location of the foreign invader cause inflammation (redness, pain, heat, swelling) as a part of the immune response. Since these cytokine messages end up causing inflammation, we also call them “pro-inflammatory cytokines.” As a consequence of the infection plus the inflammation caused by your immune system attacking the invaders, you get a painful throat, maybe a fever, and when the doctor looks at the back of the throat, they can see redness and pus (a large collection of white blood cells). This is a great example of how our own immune system not only helps us but can cause damage and misery to us as well.

After the white blood cells conquer the foreign invaders (such as the bacteria causing strep throat), we want the inflammation to stop. We do not need those white blood cells anymore in the throat. Therefore, cells start to secrete cytokines that tell the WBCs to calm down, go away, or go into retirement and die (a process called apoptosis). Since these cytokine molecular messages decrease inflammation, we call them “anti-inflammatory cytokines.”
Problem with the SLE immune system: too much proinflammatory cytokines like interferon
However, a huge problem in lupus patients is that most SLE patients with moderate to high disease activity have very high amounts of a pro-inflammatory cytokine called type 1 interferon. Type 1 interferon triggers all kinds of white blood cells (T-cells, B-cells, mononuclear cells, neutrophils, etc.) to become more active and cause inflammation throughout the body. We now consider type 1 interferon to be an important cause of lupus disease activity.
Anifrolumab works by attaching directly to type 1 interferon. This attachment prevents it from then attaching to white blood cells. Since it cannot connect to the WBCs, the WBCs do not become more active and do not cause the inflammation we see in lupus. This is the first FDA-approved drug that works in this way.
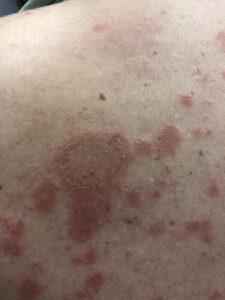
What to expect from the anifrolumab FDA approval treatment:
Many patients begin to see improvements by 2 months’ treatment. It especially appears useful for moderate to severe cutaneous (skin) lupus in decreasing active inflammation, redness, tenderness, and itching. In the clinical trials, 49% of those with at least moderately severe cutaneous lupus had at least a 50% disappearance of skin inflammation by 3 months compared to 25% of those receiving placebo (they were actually on the usual standard of care treatments). That is a remarkable difference.
NOTE: See my previous blog post about Saphnelo to see an example of how well it works on skin lupus.
Fortunately, it can significantly reduce lupus inflammatory arthritis pain and swelling. It also improves low blood cell counts due to SLE. Immune system activity markers of lupus (anti-dsDNA, C3, and C4) tend to improve. It can decrease the number of and frequency of lupus flares and help reduce the need for steroids.
The clinical trials did not include patients with severe kidney inflammation (lupus nephritis) and neuropsychiatric (brain and nerve) lupus. Therefore, we do not know how these patients would do on anifrolumab.
Interferon levels are high in other related disorders such as scleroderma, dermatomyositis, and Sjogren’s. Experts hope that it may be beneficial in these, but it has not been studied as of today.
Please remember to do all other recommended measures to control your lupus. If you go on Saphnelo, make sure you learn and abide by The Lupus Secrets as well.

How anifrolumab is taken: 300 mg of anifrolumab is given intravenously (IV) monthly.
Alcohol/food interactions with anifrolumab: No known food or alcohol interactions.
Potential side effects of anifrolumab:
Interestingly, anifrolumab caused fewer severe side effects in the clinical trials than the “placebo” group. On the surface, this may not make sense. However, the studies compared SLE patients with active disease who took their usual medicines for lupus (called standard of care, SOC) plus anifrolumab versus patients on SOC plus placebo. Therefore, the studies showed that when anifrolumab was added to medicines we usually use to treat lupus, they had fewer severe side effects than those taking SOC. One possible reason for this is that steroids are the number one cause of serious drug side effects in SLE patients. Those who took anifrolumab could significantly lower their doses of this dangerous (but necessary) lupus drug.
Fortunately, there were few severe infections and opportunistic infections (such as tuberculosis and pneumocystis pneumonia) in the patients treated with anifrolumab.
Anifrolumab-treated patients developed shingles more often
However, we do need to exercise some caution. Interferon is an essential immune system molecule (cytokine) crucial in fighting off viral infections and cancer. Patients taking anifrolumab were 3 times more likely to develop shingles of mild to moderate severity than those in the placebo (standard of care) group. Fortunately, no severe shingles attacks occurred.
Yet, a big question is, what happens in the long run? These studies were only conducted for 1 to 3 years each. Will patients on anifrolumab develop more (or worse) viral infections such as the flu, hepatitis B, hepatitis C, and coronavirus (COVID-19)? There were a small number of increased influenza cases in patients taking anifrolumab in the clinical trials. However, this was only a small number of patients. More patients on anifrolumab had upper respiratory tract infections (URI, like the common cold), bronchitis, and pharyngitis (throat infection). These are most commonly due to viral infections.
Currently, as of August 2021, the longest study was a 3-year extension trial from one of the phase 2 clinical trials. Even in this study, severe side effects were uncommon. Amazingly, only 7% of the patients stopped anifrolumab due to side effects. In conclusion, 93% of patients tolerated anifrolumab well and continued taking it for the entire 3 years.
Get all Vaccines to Prevent the Vast Majority of Saphnelo Side Effects
This list of side effects seen in 2% or more of patients in the Saphnelo clinical trials is ridiculously few. Most of them deal with viral infections (type 1 interferon is essential for fighting off viral infections).
Shingles is an excruciating rash due to the chickenpox virus. People with systemic lupus erythematosus (SLE) are already three times more likely to get shingles than older healthy people.
The vast majority of those treated with Saphnelo did not get shingles. However, five times more Saphnelo patients got shingles than those treated with placebo.
I recommend all my patients get a shingles vaccine (preferably Shingrix) before starting Saphnelo. The Shingrix vaccine is 95% effective at preventing shingles.
Since infections are the most common side effects seen with Saphnelo, the best thing people can do to avoid Saphnelo side effects is get vaccinated against them. For example, no properly vaccinated patients got shingles or COVID in the Saphnelo clinical trials. Therefore, strongly consider getting all of the following:
- Shingrix (at least one dose before starting Saphnelo)
- COVID vaccine every Fall/Winter. As of 2025, the CDC recommended that immunocompromised patients (SLE and Saphnelo patients) should get a 2nd shot (a booster of that year’s COVID vaccine) 2-6 months after their 1st COVID vaccine of the season.
- Influenza (flu) shot every September/October
- Prevnar-21 or Prevnar-20 (to reduce the risk of invasive pneumococcal infections and pneumonia)
- Gardasil series ( to reduce the risk of human papillomavirus infections and its related cancers, which occur more commonly in SLE patients)
- RSV vaccine (though most insurances will not pay for it until patients are 60 and older, but hopefully this will change in the future)
- Hepatitis B vaccine series
Could the anifrolumab FDA approval cause more cancers?
Thus far, as of 2025, we have seen no increases in any cancers with Saphnelo.
Only time will tell. I will recommend that my patients considering anifrolumab be vaccinated against shingles, hepatitis A, hepatitis B, and influenza. They should also stay up to date on all cancer screenings and develop good habits to decrease their risk for infections and cancers.
Being a new biologic, it will most likely be expensive.
What needs to be monitored while taking anifrolumab:
Your doctor will check the usual labs to follow lupus patients while you are on the treatment.
Reasons not to take anifrolumab (contraindications or precautions):
Do not take anifrolumab if you have an active infection. Patients should be checked for HIV, hepatitis B, and hepatitis C infection before starting treatment.
Patients with immune deficiency disorders, HIV, viral hepatitis B or C, or a history of severe herpes infections should not take anifrolumab.
While taking anifrolumab:
If you develop any infection symptoms at all, such as fever, sore throat, cough, red, painful skin, sinusitis congestion, or painful, frequent urination, see your primary care doctor or go to an urgent care center immediately. Do not get your anifrolumab treatment if you have an active infection. You should not receive live vaccines, including Zostavax (for shingles) while being treated with anifrolumab.

Vaccines and anifrolumab:
At this time, we do not know the vaccine recommendations with the anifrolumab FDA approval yet. Ask your doctor. I plan to recommend my patients get inactivated (non-live) vaccines such as the flu shot, pneumonia shots, COVID-19 vaccines, and the Shingrix shingles shot while on anifrolumab. However, we do not know if anifrolumab should be stopped around the time of getting vaccinated or not.

Pregnancy and breast-feeding:
Do not use anifrolumab while pregnant or breast-feeding. Since anifrolumab is a large molecule, very little of it probably gets into breast milk. However, we need to await future studies and recommendations.
Geriatric use of anifrolumab:
Anifrolumab has not been studied in a large number of older people. Yet, there may be an increased risk of side effects such as infections as may occur with other immunosuppressant medicines in older people.
What to do with anifrolumab at the time of surgery:
However, the effects of anifrolumab in patients undergoing surgery have not been studied. In patients whose lupus is under excellent control, we may recommend they delay elective surgeries (such as joint replacement surgery) for a couple of months after anifrolumab (skipping one anifrolumab treatment during that time), then resuming anifrolumab a couple of weeks after surgery if there are no infection problems. This would be a reasonable approach in the absence of studies to guide us. However, discuss with your doctors.
Learn more about Saphnelo and get patient assistance:
Patient assistance program for anifrolumab: saphnelo
Drug helpline for anifrolumab: saphnelo
Website to learn more about anifrolumab: to learn more about the anifrolumab FDA approval
AUTHOR:
Don Thomas, MD, author of “The Lupus Encyclopedia” and “The Lupus Secrets”
References:
Parts of the above come from a manuscript that is in preparation as of July 2021 for chapter 34 (FDA Approved Lupus Drugs) for the 2nd edition of “The Lupus Encyclopedia” by Johns Hopkins University Press (JHUP). None of the above has been reviewed by the editors of JHUP. It is not meant to replace the advice and recommendations of your healthcare providers. The information on drugs changes as newer research studies are done. Readers should double-check any information with their healthcare provider.
Chatham WW, Furie R, Saxena A, Brohawn P, Schwetje E, Abreu G, Tummala R. Long-Term Safety and Efficacy of Anifrolumab in Adults With Systemic Lupus Erythematosus: Results of a Phase II Open-Label Extension Study. Arthritis Rheumatol. 2021 May;73(5):816-825. doi: 10.1002/art.41598. Epub 2021 Mar 24. PMID: 33225631; PMCID: PMC8252065.
Felten R, Scher F, Sagez F, Cohasset F, Arnaud L. Spotlight on anifrolumab and its potential for the treatment of moderate-to-severe systemic lupus erythematosus: evidence to date. Drug Des Devel Ther. 2019 May 8;13:1535-1543. doi: 10.2147/DDDT.S170969. PMID: 31190735; PMCID: PMC6514126.
Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, Illei GG, Drappa J, Wang L, Yoo S; CD1013 Study Investigators. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017 Feb;69(2):376-386. doi: 10.1002/art.39962. PMID: 28130918; PMCID: PMC5299497.
Furie RA, Morand EF Bruce IN, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomized, controlled, phase 3 trial. Lancet Rheumatol. 2019; 1 : e208-e219
Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, Bae SC, Brohawn PZ, Pineda L, Berglind A, Tummala R; TULIP-2 Trial Investigators. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med. 2020 Jan 16;382(3):211-221. doi: 10.1056/NEJMoa1912196. Epub 2019 Dec 18. PMID: 31851795.
Further References
Niewold, T. Targeting type I interferon in systemic lupus erythematosus. Nat Rev Rheumatol 12, 377–378 (2016). https://doi.org/10.1038/nrrheum.2016.83
Oke, V., Iva Gunnarsson, Jessica Dorschner, Susanna Eketjäll, Agneta Zickert, Timothy B. Niewold & Elisabet Svenungsson. High levels of circulating interferons type I, type II, and Type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Res Ther 21, 107 (2019). https://doi.org/10.1186/s13075-019-1878-y
Paredes JL, & Timothy B. Niewold (2020) Type I interferon antagonists in clinical development for lupus, Expert Opinion on Investigational Drugs, 29:9, 1025-1041, DOI: 10.1080/13543784.2020.1797677
Postal M, Vivaldo JF, Fernandez-Ruiz R, Paredes JL, Appenzeller S, Niewold TB. Type I interferon in the pathogenesis of systemic lupus erythematosus. Curr Opin Immunol. 2020 Dec;67:87-94. doi: 10.1016/j.coi.2020.10.014. Epub 2020 Nov 24. PMID: 33246136; PMCID: PMC8054829.
Tanaka Y, Tummala R. Anifrolumab, a monoclonal antibody to the type I interferon receptor subunit 1, for the treatment of systemic lupus erythematosus: an overview from clinical trials. Mod Rheumatol. 2021 Jan;31(1):1-12. doi: 10.1080/14397595.2020.1812201. Epub 2020 Sep 17. PMID: 32814461.
For more in-depth information on Anifrolumab FDA Approval to Treat Systemic Lupus [Saphnelo February 2025 Update]:
Read more in The Lupus Encyclopedia, edition 2
Look up your symptoms, conditions, and medications in the Index of The Lupus Encyclopedia
If you enjoy the information from The Lupus Encyclopedia, please click the “SUPPORT” button at the top of the page to learn how you can help.
What are your comments and opinions?
If you have lupus, what has your experience been? What do you recommend for other patients?
Do you have any questions to ask Dr. Thomas?
Please click on “Leave a Comment” above to comment.
Please support “The Lupus Encyclopedia” blog post page
Click on “SUPPORT” at the top of the page to learn how you can support “The Lupus Encyclopedia“
10 Comments
 Gaile
Gaile donthomasj@aol.comModerator
donthomasj@aol.comModeratorGaile: Good luck! I hope you and your rheumatologist are able to find a treatment regimen that helps you out better! … Don Thomas, MD
 BRIDGET SIMPSON
BRIDGET SIMPSONThis sounds so much better for me than Benlysta. I’m excited that it could reduce my ever climbing inflammation & eliminate steroids
 donthomasj@aol.comModerator
donthomasj@aol.comModeratorBridget: If you go on anifrolumab, we would love to hear how you do and what problems it helped or did not help you with… Dr T
 Denise
DeniseHow could you find out if this will be approved in Canada?
 donthomasj@aol.comModerator
donthomasj@aol.comModeratorDenise: Anifrolumab was submitted to Health Canada in December2020: When belimumab (Benlysta) was FDA approved in 2011 in the US, Canada followed suit just three months later. Since the anifrolumab FDA approval was July 2, 2021, wouldn’t it be wonderful if it were approved by Health Canada around September? fingers crossed … Dr T
 Donna D.
Donna D.I’m just wondering if I’m a candidate for this treatment. My inflammation numbers are within normal range, but my wbc are low. The lowest they’ve been is 1.3. My rheumatologist is happy when they are in the 2 range. My platelets range from low normal to slightly low.
 donthomasj@aol.comModerator
donthomasj@aol.comModeratorDonna: It is always a very personal, clinical call, putting all of the pieces together. Many patients with SLE can have bad active disease, yet have perfect labs. The labs, to include the inflammatory markers, do not measure what is going on in the joints and skin for example. In many patients they do (for example, low C3/C4, or high anti-dsDNA or high EC4d/ESR/gammaglobulins or CRP), and it makes it much easier for us docs to help monitor disease activity. For example, one of my patients has horribly active cutaneous lupus right now, and she voiced her concern on her labs show no inflammation. I had to explain to her that although the labs do not, if we were to do a biopsy of her inflamed skin, it would show a ton of inflammation and over active immune system. However, I know she would really hate me if I did a biopsy of her skin regularly to monitor the inflammation. Lupus is a strange and difficult disease! Thanks for your question and good luck! Bottom line = if you are not in remission or low disease activity, it may be a good treatment for you to discuss with your doctor. … Donald Thomas, MD
 Anne Kirlin
Anne KirlinI have a hard time seeing where the new updates are- I see that this says updated February 2025, but I don’t see what has been updated
Thanks Donald Thomas, MDModerator
Donald Thomas, MDModeratorAnne: I added recommendations on preventing infections since Saphnelo has quite a low number of significant side effects and the vast majority are infections, most of which are preventable:
Saphnelo increases the risk of shingles and other viral infections
This list of side effects seen in 2% or more of patients in the Saphnelo clinical trials is ridiculously few. Most of them deal with viral infections (type 1 interferon is essential for fighting off viral infections).Shingles is an excruciating rash due to the chickenpox virus. People with systemic lupus erythematosus (SLE) are already three times more likely to get shingles than older healthy people.
The vast majority of those treated with Saphnelo did not get shingles. However, five times more Saphnelo patients got shingles than those treated with placebo.
I recommend all my patients get a shingles vaccine (preferably Shingrix) before starting Saphnelo. The Shingrix vaccine is 95% effective at preventing shingles.
Since infections are by far the most common side effects seen with Saphnelo, the best thing people can do to avoid Saphnelo side effects is to get vaccinated against them. For example, no properly vaccinated patients got shingles or COVID in the Saphnelo clinical trials. Therefore, strongly consider getting all of the following:
Shingrix (at least one dose before starting Saphnelo)
COVID vaccine every Fall/Winter. As of 2025, the CDC recommended that immunocompromised patients (SLE and Saphnelo patients) should get a 2nd shot (a booster of that year’s COVID vaccine) 2-6 months after their 1st COVID vaccine of the season.
Influenza (flu) shot every September/October
Prevnar-21 or Prevnar-20 (to reduce the risk of invasive pneumococcal infections and pneumonia)
Gardasil series ( to reduce the risk of human papillomavirus infections and its related cancers, which occur more commonly in SLE patients)
RSV vaccine (though most insurances will not pay for it until patients are 60 and older, but hopefully this will change in the future)
Hepatitis B vaccine series




Leave a comment