Any trials in Canada?
CAR-T Cell Therapy For Lupus Made Simple (January 2025 UPDATE)
The field of CAR-T therapies in treating autoimmune diseases like lupus is rapidly expanding. As of April 2024, at least 43 patients with severe systemic lupus erythematosus (SLE) had been treated with CAR-T cell therapy. Most went into drug-free remission, meaning they were completely off their lupus medications (like prednisone, hydroxychloroquine, methotrexate, mycophenolate, etc). There were very few major side effects.
_____________________
NOTE: Johns Hopkins University Press, publisher of The Lupus Encyclopedia, is a nonprofit publisher. If you purchase JHUP books, like The Lupus Encyclopedia, you support projects like Project MUSE.
_____________________
January 2025 Update: We need patients for CAR-T Studies, Please!
The world of CAR-T has EXPLODED! There were so many research papers, presentations, meetings about CAR-T during our November 2024 American College of Rheumatology meeting that I self-named the meeting: “The CAR-T Meeting” and a new chapter in the treatment of lupus.
Currently, patients with severe SLE can apply for many ongoing clinical trials with the help of their rheumatologist, and researchers will start many more soon. This truly is the new chapter in the treatment of autoimmune diseases.
If you are interested in participating in a clinical trial, go to the following websites, get the information, and give it to your rheumatologist to find out if you are eligible:
https://www.cabalettabio.com/patients/phase-12-trial-in-lupus CabalettaBio
https://www.mayo.edu/research/clinical-trials/cls-20513089 Mayo Clinic
https://www.bmsclinicaltrials.com/us/en/clinical-trials/NCT05869955?cart-autoimmune=true Bristol Myers Squibb
https://ir.cartesiantherapeutics.com/news-releases/news-release-details/cartesian-therapeutics-highlights-progress-and-2025-strategic Cartesian Therapeutics
https://www.novartis.com/clinicaltrials/study/nct05798117 Novartis
https://ir.allogene.com/news-releases/news-release-details/allogene-therapeutics-secures-us-fda-ind-clearance-allo-329 Allogene
For systemic lupus and lupus nephritis click here, or copy and paste the following: https://clinicaltrials.gov/search?cond=lupus&term=CAR-T
Also click on the Lupus Foundation’s Clinical Trials site or copy and paste the following: https://antidote.me/match/search/questions/1?utm_source=lupus_org&utm_medium=ctsearch&utm_campaign=unisearch
- For Sjogren’s disease click here or copy and paste the following: https://clinicaltrials.gov/search?cond=sjogren%27s&term=CAR-T
- For rheumatoid arthritis click here or copy and paste the following: https://clinicaltrials.gov/search?cond=rheumatoid&term=CAR-T
- For myasthenia gravis click here or copy and paste the following: https://clinicaltrials.gov/search?cond=myasthenia%20gravis&term=CAR-T
- For autoimmune diseases in general click here or copy and paste the following: https://clinicaltrials.gov/search?cond=autoimmune&term=CAR-T
- For multiple sclerosis click here or copy and paste the following: https://clinicaltrials.gov/search?cond=multiple%20sclerosis&term=CAR-T
___________________
NOTE TO READERS: CAR-T cell therapy for autoimmune diseases is a rapidly growing field. If you know of any studies I am missing, please let me know in the comments
If you know of a study accepting patients that is not listed, please put the link into a COMMENT for me to add to the list
___________________________________
4/27/24 Update
An “off-the shelf” version of CD-19 CAR-T is now recruiting SLE patients (Nebraska/Minnesota)
4/11/24 Update:
It appears that the number of patients being treated with CAR-T is accelerating (this up date is up to 43 SLE patients treated with CAR-T). From this point onwards, I’m sure I won’t be able to include all study releases. However, if you know of any that I miss, please reply in the comments with a link to the study so that I can include that data.
Several sites are now recruiting for SLE patients, see below.
In addition to SLE, I will discuss these autoimmune diseases that have responded to CAR-T therapy:
– systemic sclerosis (scleroderma)
3/24/24 Update:
- At least 43 SLE patients had been treated as of March 2024
- Update on the 15 German autoimmune disease patients
- Novartis reports success in 6 SLE patients
- Two pediatric lupus nephritis patients treated with CAR-T
- Two SLE patients with lupus nephritis treated with Kyverna’s KYU-101
- 12 patients with neuromyelitis optica do well with BCMA
- Two Sjogren’s disease patients and one rheumatoid arthritis patient responded well to CAR-T cell therapy
OTHER LINKS TO CAR-T STUDIES ARE AT THE END OF THIS ARTICLE
——————–
1/17/24 Update: CAR-T lymphomas seen after some CAR-T cell therapies. Discussed below (click on the highlighted link above)
—————————————-
Is CAR-T a miracle cure for lupus? Is this the future and the start of a new era?
From 2021 to 2023, doctors at the Friedrich-Alexander University, Erlangen-Nürnberg, Erlangen, Germany, described 8 patients with treatment-resistant severe systemic lupus erythematosus who went into remission using a treatment called CAR-T cell therapy. The CAR-T treatment was safe, caused no major side effects, and all the patients stayed in remission without additional ongoing medications or immunosuppressants. CAR-T therapy has kept the initially treated patient in remission for over 3 years. The 2024 EULAR meeting in Vienna, Austria, in June 2024, showcased the data on her long-lasting remission. Two SLE patients in China are still in remission after CAR-T cell therapy treatment over 3 1/2 years ago.
Since then, CAR-T cells have been a hot topic among patients with autoimmune diseases and rheumatologists. However, most people do not precisely understand CAR-T cells.
The purpose of this blog post is to simplify the concept of CAR-T cells.
I’ll start with a much-simplified explanation. I recommend that you read at least this initial short, easy-to-understand section.
I’ll then take a simplified deep dive into the science of CAR-T cell therapy for lupus.
I’ll talk about CAR-T therapy’s pros, cons, and future.
Then, I’ll list the clinical trials that are going on as of April 2024 (the number of research studies continues to grow since then). The number of new clinical trials is rapidly increasing. If you know of any new ones I have not included, please let me know in the Comments section (click on “Leave a Comment” top right of this page).
Doctors can refer patients to some of these for CAR-T therapy for lupus. I have included the contact information as well.
What are CAR-T cells?
CAR-T cells are specially engineered T-cells (a type of white blood cell) infused into the patient. These CAR-T cells identify B-cells (a type of white blood cell described below) in the patient’s body to attack and destroy those B-cells.
This is important in lupus because the B-cells make the antibodies (called autoantibodies, like anti-dsDNA) that attack and destroy the patient’s body organs. Destroying these “bad” B-cells (scientifically called “autoreactive B-cells”) results in the disappearance of these organ-destroying antibodies.
CAR-T cells is short for “chimeric antigen receptor T-cells.” We can break this down into four parts (chimeric, T-cells, and the antigen receptor that is produced by virus-inserted DNA):
Chimeric means something that is made up of different organisms. The chimera was a Greek monster that was part lion, goat, and snake.

CAR-T cells explained
The Patient’s T-cells
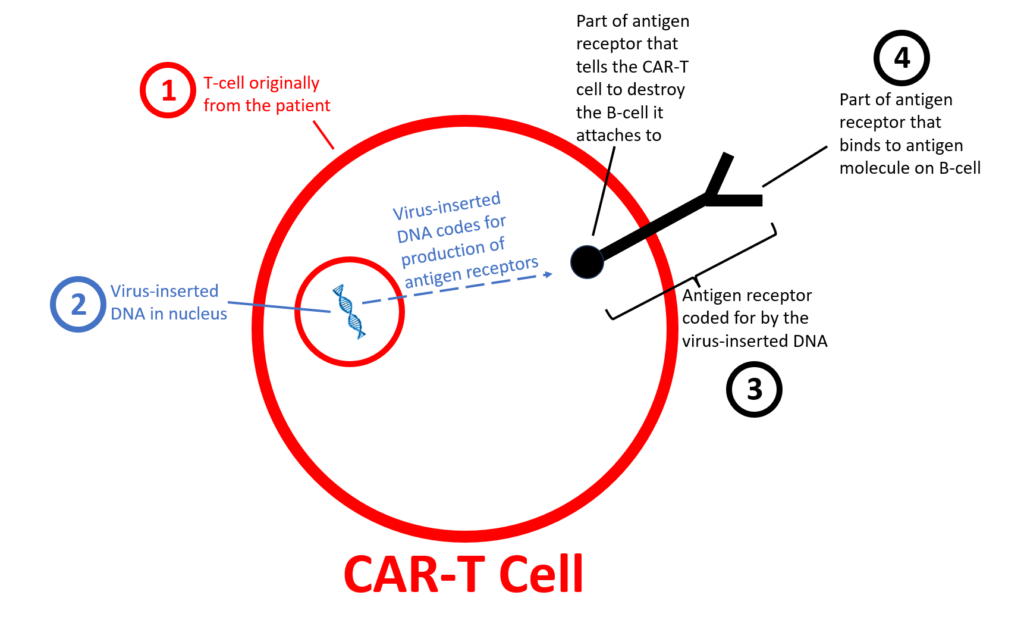
T cells (① in the diagram below) come from the patient themselves. These T-cells from the patients are the primary sources of the live CAR-T cells.
An antigen receptor (③) is produced by genes (②) that a virus inserts into the inner nucleus of the T-cell.
Therefore, a chimeric antigen receptor T-cell (CAR-T) is made of the cells originally from the patient but also has foreign genetic DNA introduced by a virus. This foreign DNA produces “antigen receptors” that become inserted in the T cells’ outer membranes.
The Antigen Receptors
Antigen receptors (③ in the diagram above) are produced by virus-inserted DNA and inserted into the T-cell’s outer membrane.
The antigen receptor has two main sections: an outer section outside the cell and an inner section inside the cell. The antigen receptor’s outer section (sticking outside of the CAR-T cell) can identify and attach to antigens.
Think of an “antigen” as an identification badge. In this case, the antigens are molecules found on B-cell surfaces. The antigen used in the 8 SLE patients described above was CD-19, which is only found on mature B-cells (but not on plasma cells). The CD-19 antigen identifies that cell as a B-cell, not any other type (like a T-cell, muscle cell, or red blood cell).
The antigen receptor’s outer section identifies and attaches to other cell’s antigens (on the outer membrane of that other cell, in this case, B-cells). This attachment to the other cell’s antigen signals the antigen receptor’s inner portion to tell the T-cell to destroy the cell (B-cell) it is attached to.
B-Cells and Lupus
B-cells are white blood cells of the immune system that make the dangerous autoantibodies of lupus. For example, the B-cells of many lupus patients make an autoantibody called “anti-dsDNA.” “Auto-“ comes from the Greek word for “self.” Autoantibodies are immune system molecules that attack the person itself. B-cells that make lupus autoantibodies that attack the person’s own self are called “autoreactive B-cells.”
I’ll simplify this in this article and call autoreactive B-cells “bad B-cells.”
Autoantibodies (like anti-dsDNA) can go to the organs (like the kidneys) causing inflammation, damage, and even organ destruction. When they attack the kidneys, this is called lupus nephritis. Destroying these “bad” B-cells could potentially help decrease this inflammation and damage.
The antigen receptors on the CAR-T cells identify the patient’s B-cells and attach to the B-cells. This activates the part of the antigen receptor inside the CAR-T cell, which tells the CAR-T cell to destroy the B-cell that it is attached to.
The CAR-T cells are finally produced for lupus patients
Laboratory technicians finally produce the CAR-T cells in a laboratory by infecting the patient’s T-cells (① in the diagram above) with virus that are designed to insert DNA (② in the diagram above) inside the T-cells’ nucleus. This DNA tells the patient’s T-cells to produce the antigen receptors (③ in the diagram above) described above and insert the antigen receptors into the CAR-T cell’s outer membranes.
_____________________________________________
Summary: CAR-T cells are
① T-cells that originally came from the patient being treated
② and contain virus-inserted DNA
③ that causes the production of antigen receptors on the CAR-T cell’s outer cellular membrane
④ the antigen receptors recognize and attach to B-cells so that the CAR-T cells destroy B-cells.
What is the CAR-T cell therapy process for lupus patients?
Above is a simplified explanation of CAR-T cell therapy used for lupus patients. Now, let’s have a more detailed look.
NOTE: This description is for DNA-based CAR-T cell therapies, which have been used in most patients reported on thus far. RNA-based therapies (such as Cartesian’s Descartes-08) do not involve some steps (such as using cyclophosphamide and fludarabine, and the CAR-T cells do not reproduce after being infused back inside the patient’s body).
Apheresis removes T-cells from the patient
First, the patient has blood removed by a procedure called apheresis (as in the photo below).
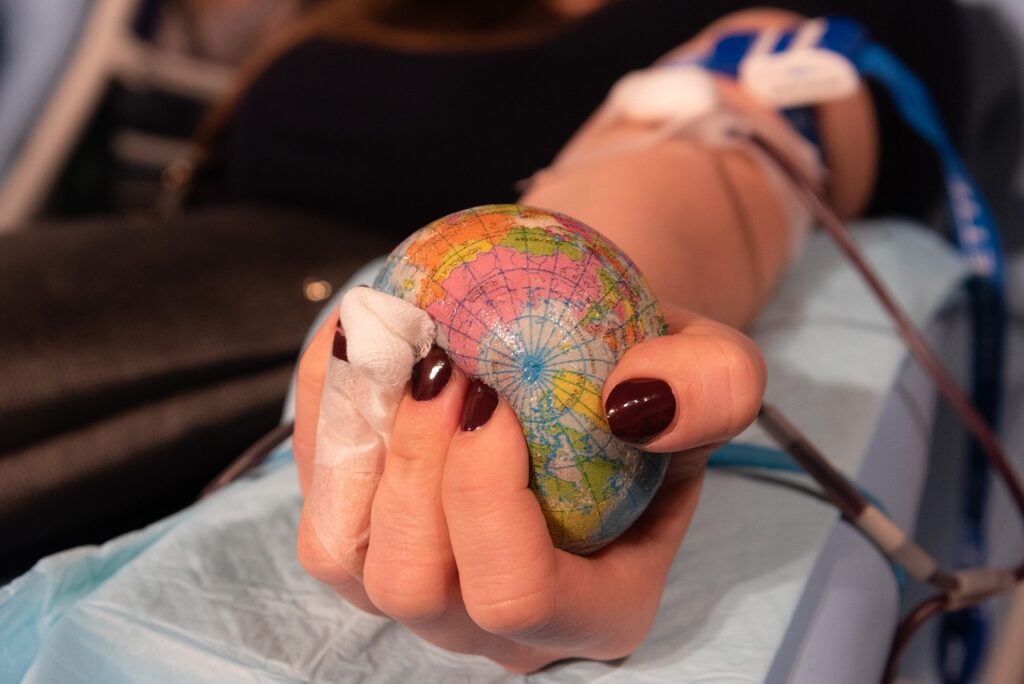
An apheresis machine (shown below) separates white blood cells (that contain the needed T-cells to turn into CAR-T cells) from red blood cells and other blood particles.
Technicians produce special DNA-inserting virus from lentivirus
Laboratories specially design a type of virus, called lentiviruses, to infect T-cells and insert DNA that makes the needed antigen receptors.
What are lentiviruses?
Lentiviruses exist in nature. They are dangerous and can be deadly. Lentiviruses especially like to infect white blood cells (like T-cells). They insert RNA (ribonucleic acid) and essential enzymes to produce DNA into the infected person’s cells. This RNA and the needed enzymes make virus DNA that it inserts into the DNA of the infected cell. This foreign, viral DNA forces the infected cell to manufacture much more lentivirus. This is how the virus reproduces itself. In the process, the cell can die. The newly constructed lentivirus particles can then infect more T-cells in the person, making them sick.
Using lentiviruses for medical treatments
Since lentivirus can insert DNA into the DNA of other cells, it is a perfect vehicle for reproducing anything that can be made from DNA (such as parts of a CAR-T cell).
For example, betibeglogene autotemcel (Zynteglo) is a gene therapy where specially engineered lentivirus inserts genes into patients’ bone marrow cells, causing them to produce healthier red blood cells. Doctors use Zynteglo to treat patients with a severe form of anemia called beta-thalassemia.
Lentivirus use in CAR-T cell therapy
For CAR-T cell production, technicians produce specially engineered lentiviruses in a laboratory. These lentiviruses do not contain their original dangerous virus-producing RNA. Instead, unique DNA (see image below) is engineered to make the needed antigen receptors for CAR-T cells. These unique DNA are placed inside lentivirus that do not infect and kill people. Instead, the engineered lentivirus can infect T-cells from a person’s body and insert this CAR-T-producing DNA.

Lentivirus inserts antigen receptor-producing DNA into the patient’s T-cells
Technicians take the white blood cells from the apheresis machine and remove as many T-cells as possible from the blood cells. In a sterile (germ-free) laboratory, these T-cells are infected with the specially engineered lentivirus DNA (or mRNA for other brands of CAR-T cells) that can tell the T-cell to produce specific antigen receptors (see image below).

The virus inserts this DNA or mRNA into the T-cells (see image below).

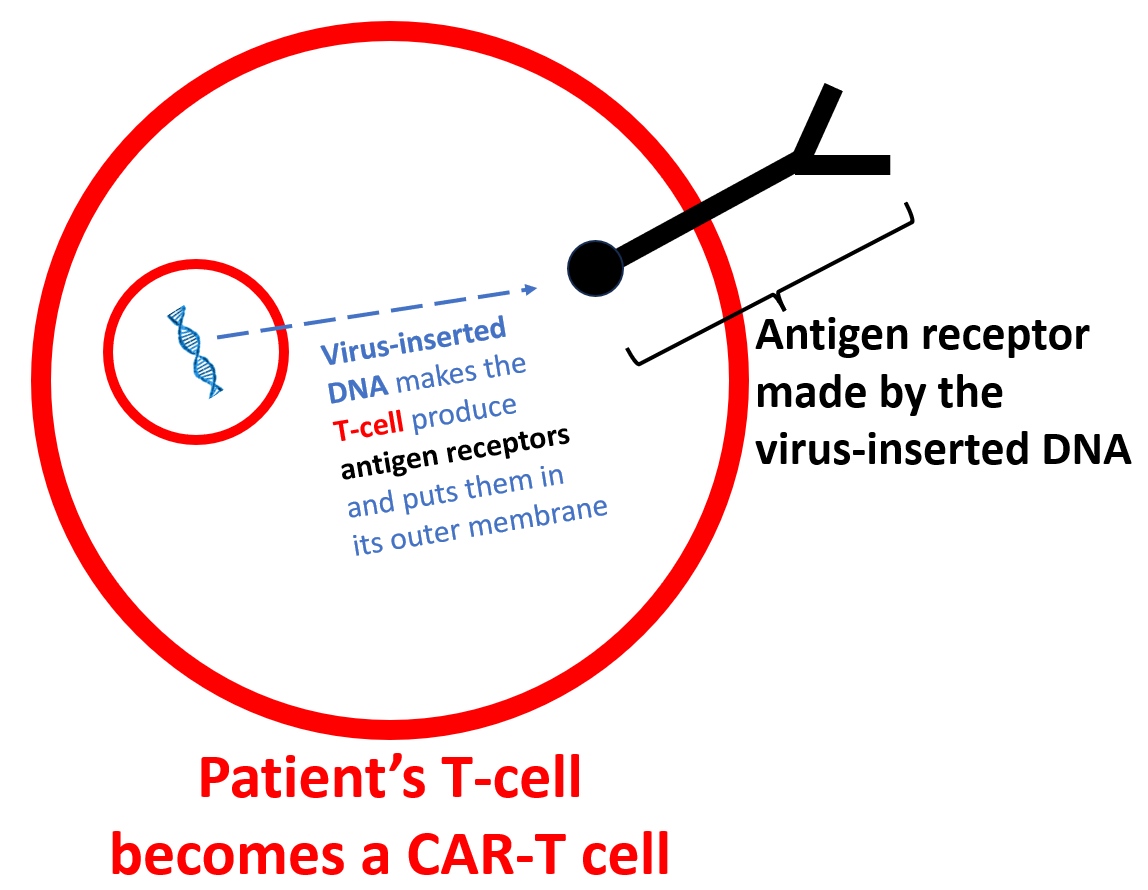
The patient’s T-cells produce the antigen receptors: A CAR-T cell is born!
The T-cells then produce the CD-19 (or BCMA, or both, but also other types are possible) antigen receptors and insert them into their outer cellular membranes (see image below). At this point, they are called CAR-T cells.
The CAR-T cells are replicated (multiply) in the laboratory
The technicians stimulate these CAR-T cells to reproduce and produce large numbers of CAR-T cells.
Chemotherapy removes T-cells (lymphodepletion) from the patient prior to CAR-T cell infusion
The patient is treated with two chemotherapy drugs: cyclophosphamide and fludarabine. These drugs cause the person’s white blood cells, especially the T-cells (called lymphodepletion), to drop significantly in number.
Note that some CAR-T cell therapies do not require chemotherapy lymphodepletion (such as Cartesian’s Descartes-08, using mRNA instead of lentivirus DNA).
Healthcare professionals infuse the CAR-T cells into the patient
The patient, who now has very few T-cells after receiving chemotherapy, is infused with the CAR-T cells produced in the lab.
The CAR-T cells multiply (replicate) inside the patient
The person’s immune system senses the loss of T-cells after the previous chemotherapy. As a natural response to this lack of T-cells, the infused CAR-T cells reproduce rapidly, trying to replace the lost numbers of T-cells in the body’s fluids and organs. This step can potentially cause side effects, like complement activation syndrome, described below.
This step occurs with CAR-T cells that are DNA-based (as in the initial 8 patients described above). CAR-T cells that are RNA-based, like Cartesian’s Descartes-08, do not multiply inside the body. This is important as there have been rare cases of DNA-based CAR-T cells resulting in CAR-T cell lymphoma (discussed below). CAR-T cell lymphomas should not occur with RNA-based CAR-T cell therapy since they do not multiply in the persons’ body.
The CAR-T cells destroy the patient’s B-cells
The CAR-T cells identify the designated antigen (in the case of the SLE patients above, this is CD-19; see image below).
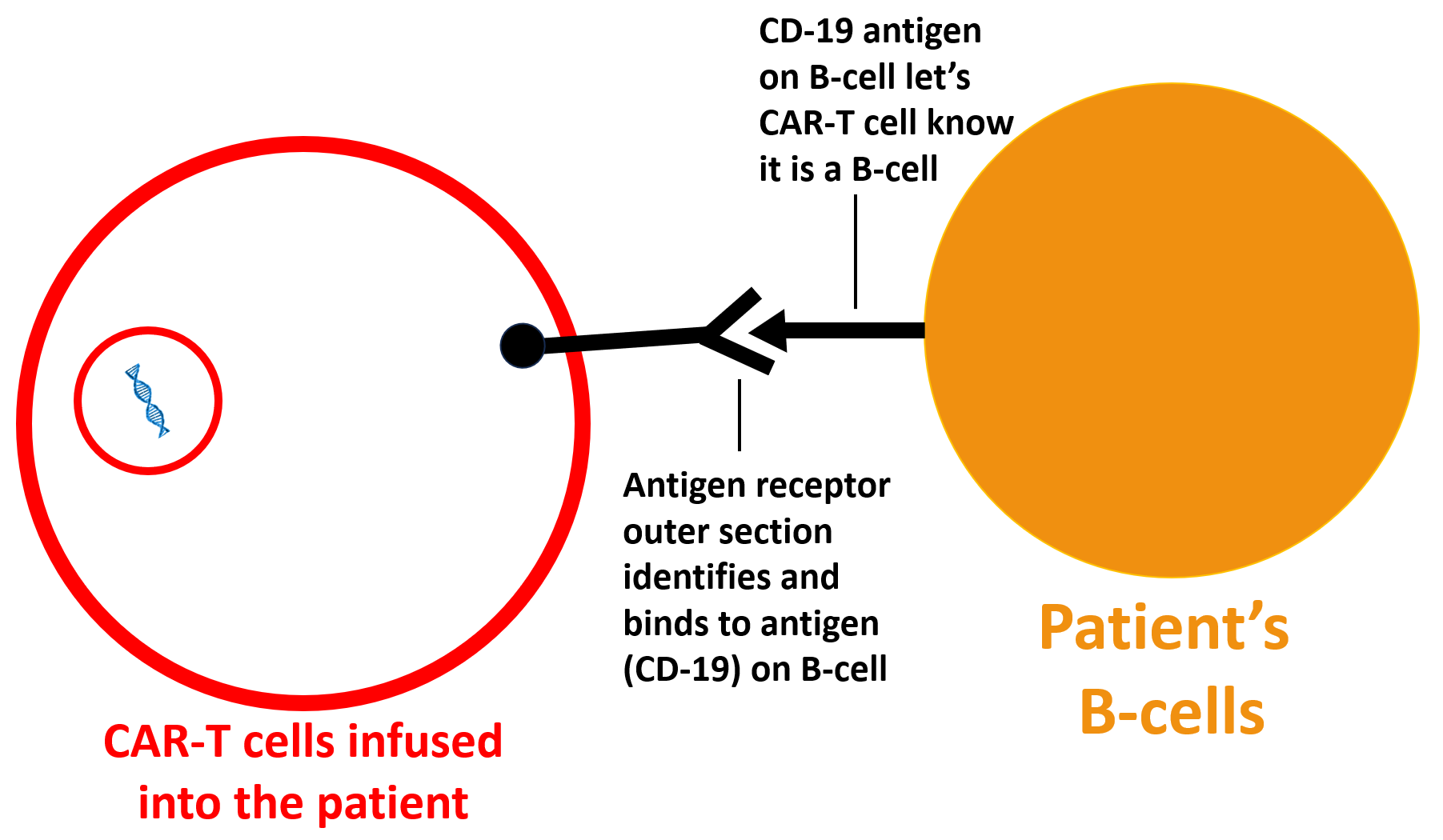
After identifying and attaching to the target antigen and cell (B-cells with CD-19 antigens on their surface), the antigen receptor activates the CAR-T cell to go into “kill mode.” (see diagram below).
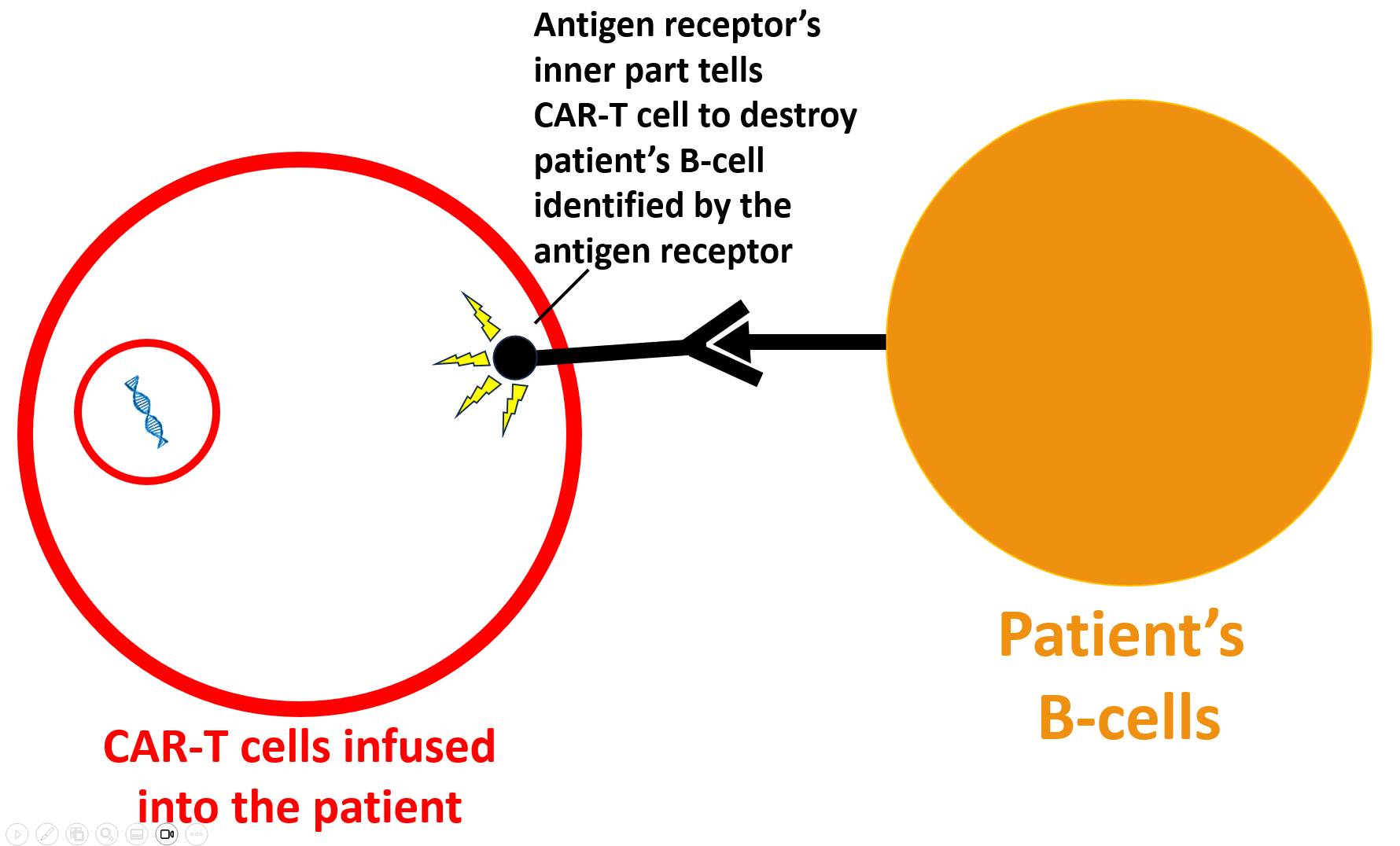
The CAR-T cell then destroys the B-cell (as per the image below). Most importantly, the CAR-T cells also destroy the “bad” B-cells that produce dangerous lupus autoantibodies (like anti-dsDNA, anti-SSA, and anti-chromatin antibodies). These dangerous antibodies disappear and stop attacking organs in the lupus patient.
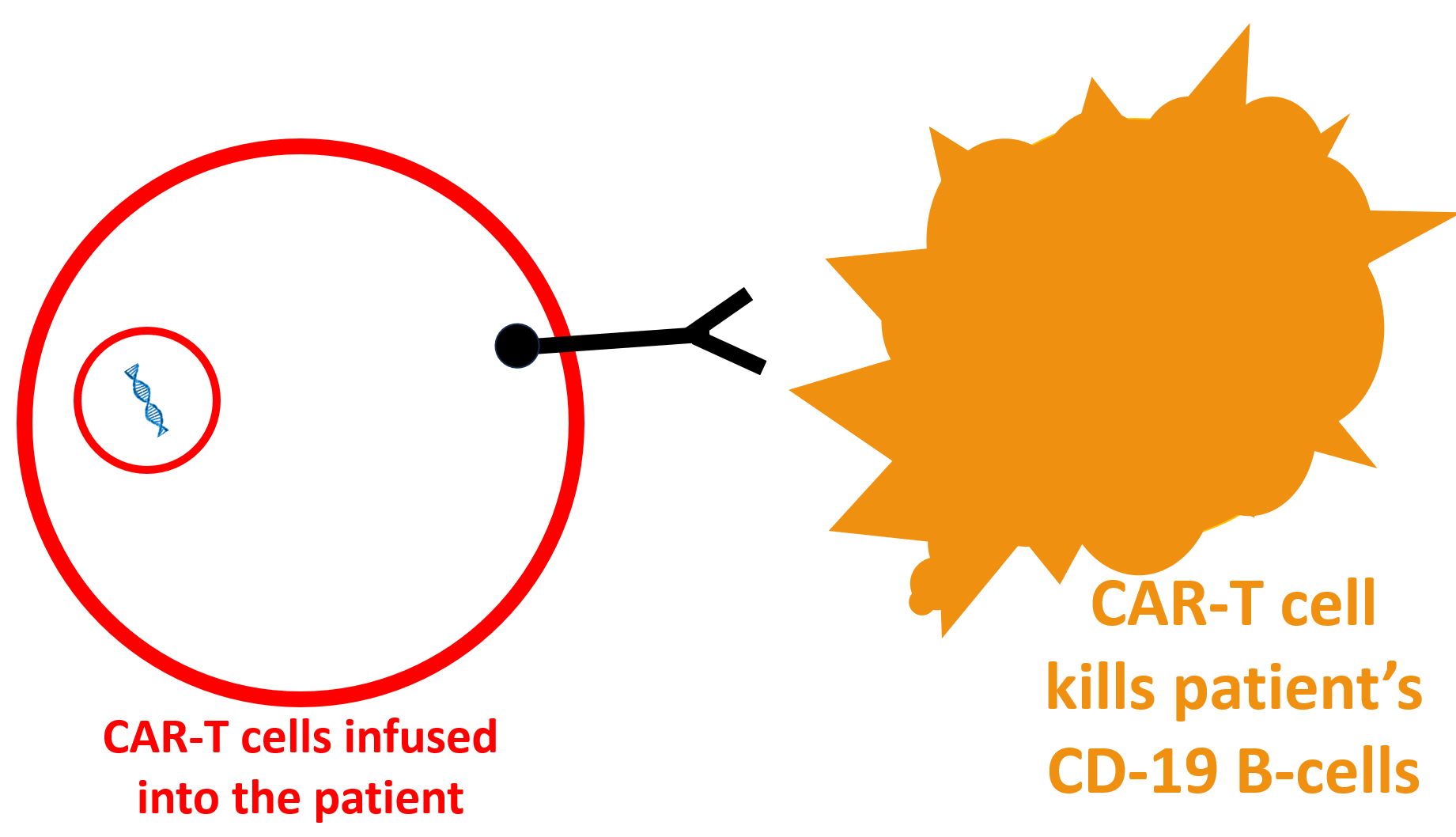
Why do CAR-T cells work better than other B-cell-depleting drugs?
Some other lupus treatments also decrease the number of B-cells (B-cell-depleting drugs). The most commonly used B-cell-depleting drugs are rituximab (Rituxan) and belimumab (Benlysta). Others, like obinutuzumab (Gazyva), also look promising. These are monoclonal antibodies produced by biological methods (i.e., biological drugs). Antibodies are also “antigen receptors,” just as in the CAR-T cells. However, they have a different structure. “Monoclonal” means that these antibody drugs are all the same type. Mono– clonal means one-clone or one-type.
They differ in which antigens they recognize. The rituximab and obinutuzumab monoclonal antibodies (also antigen receptors) recognize an antigen molecule called CD-20 found on the surface of B-cells and some T-cells. When rituximab or obinutuzumab attach to CD-20 on B-cells and some T-cells, this interaction alerts the immune system, destroying the B-cell.
The belimumab (Benlysta) monoclonal antibody (again, a type of antigen receptor) binds to an antigen called soluble B lymphocyte stimulator protein (BLyS). B-cells rely on BlyS to mature into cells that produce the dangerous lupus autoantibodies (like anti-dsDNA). However, when belimumab binds to BlyS, the BlyS molecules cannot bind to the B-cells. Therefore, the B-cells cannot mature, and their amounts decrease, reducing autoantibody production.
“Bad” autoreactive B-cells hide from B-cell-depleting drugs
Although these drugs work in many lupus patients, they do not help many others. One probable reason is that these drugs cannot reduce all B-cells in the body. These monoclonal antibody drugs are present in the body’s fluids (blood and lymph) and work on the B-cells in these fluids. However, there are a large number of B-cells producing dangerous lupus autoantibodies in the tissues of the body where these monoclonal antibodies are unable to reach. For example, research studies show that B-cells continue to reside in the joints, lymph nodes, and tonsils after treatment with these agents.
CAR-T cells find and destroy all B-cells
One advantage of CAR-T cells is that they easily penetrate the tissues (like the joints and lymph nodes), where they can attack those B-cells unaffected by belimumab, rituximab, and obinutuzumab. The B-cell destruction is complete. This differs from monoclonal antibody therapies (rituximab, belimumab, obinutuzumab), where dangerous, “bad” B-cells still exist in the body’s tissues.
Rituximab and obinutuzumab target CD-20 antigens on the cell surface of B-cells and some T-cells. One problem with this is that a type of mature B-cell called plasmablasts does not have CD-20 antigens, and they persist after rituximab treatment. Plasmablasts produce dangerous autoantibodies, like anti-dsDNA, and are increased during lupus flares in rituximab-treated patients. CAR-T cells destroy these plasmablasts (a type of mature B-cell).
Another disadvantage of monoclonal antibody drugs (like rituximab and belimumab) is that patients must continuously use them. When stopped, the “bad” B-cells that produce the dangerous autoantibodies increase again.
Side effects of CAR-T cell therapy for lupus
Fortunately, CAR-T cell therapy in patients with lupus caused few major side effects in the published medical studies (as of October 2024).
This is not what happens in patients with cancer treated with CAR-T cells. Blood cell cancers, where the B-cells become cancerous, have been the primary use for CAR-T cell therapy. Several FDA-approved CAR-T treatments are available to treat B-cell leukemia and multiple myeloma (a cancer of plasma cells, which are mature B-cells). In these studies, some severe side effects and even deaths occurred.
We must consider these possible side effects when treating autoimmune diseases. However, there are some significant differences between B-cell cancers and autoimmune diseases.
One of the most important is the number of B-cells killed by the CAR-T cells. Much lower numbers of B-cells are destroyed in autoimmune disease patients. However, huge numbers of B-cells are destroyed in people who have B-cell cancers and multiple myeloma. For example, in the first few weeks of CAR-T cell therapy for B-cell cancer, a few pounds worth of B-cells can be destroyed. This large number of B-cell deaths could be a reason for some of the severe side effects seen when CAR-T is used in cancer patients, yet not seen in patients with autoimmune disease.
Cytokine release syndrome (CRS)
One side effect is called “cytokine release syndrome” (CRS). You may remember hearing this term during the COVID-19 pandemic. It was also called “cytokine storm.” COVID-19 infection could cause CRS and was a common reason for deaths. CRS is a condition where the immune system becomes overactive, attacks the person’s organs, and can lead to death. Common symptoms of CRS include fever, body pains, headaches, lightheadedness, and drops in blood pressure.
The same thing can happen with CAR-T therapy for B-cell cancers. Some immune system molecules (like IL-6) are exceptionally high during CRS. Tocilizumab (Actemra) inhibits the activity of IL-6 and is FDA-approved to treat some autoimmune diseases, like rheumatoid arthritis. Also, it effectively treats CRS caused by CAR-T therapy and COVID-19. Steroids are also used to treat CRS. Using tocilizumab and steroids, most people survive CRS.
Most autoimmune disease patients have developed CRS. Fortunately, no severe, untreatable CRS occurred in any SLE patients. So far, it has been a manageable side effect.
Immune effector cell-associated neurotoxicity syndrome (ICANS)
Immune effector cell-associated neurotoxicity syndrome (ICANS) is another dreaded side effect of CAR-T therapy. ICANS affects the brain and nerve cells, potentially causing permanent damage if severe. Common symptoms of ICANS include headaches, tremors, tingling sensations, coordination problems, and moodiness. More severe complications include muscle weakness, numbness, trouble speaking and writing, confusion, hallucinations, seizures, coma, and even death. Major risk factors for developing ICANS are having a high number of B-cell cancer cells and if the person had had any previous neurologic problems.
As of October 2024, ICANS has not been seen in SLE patients treated with CAR-T (in the studies that I am aware of).
______________________________________________________________________________
CAR-T Cell Lymphoma after CAR-T
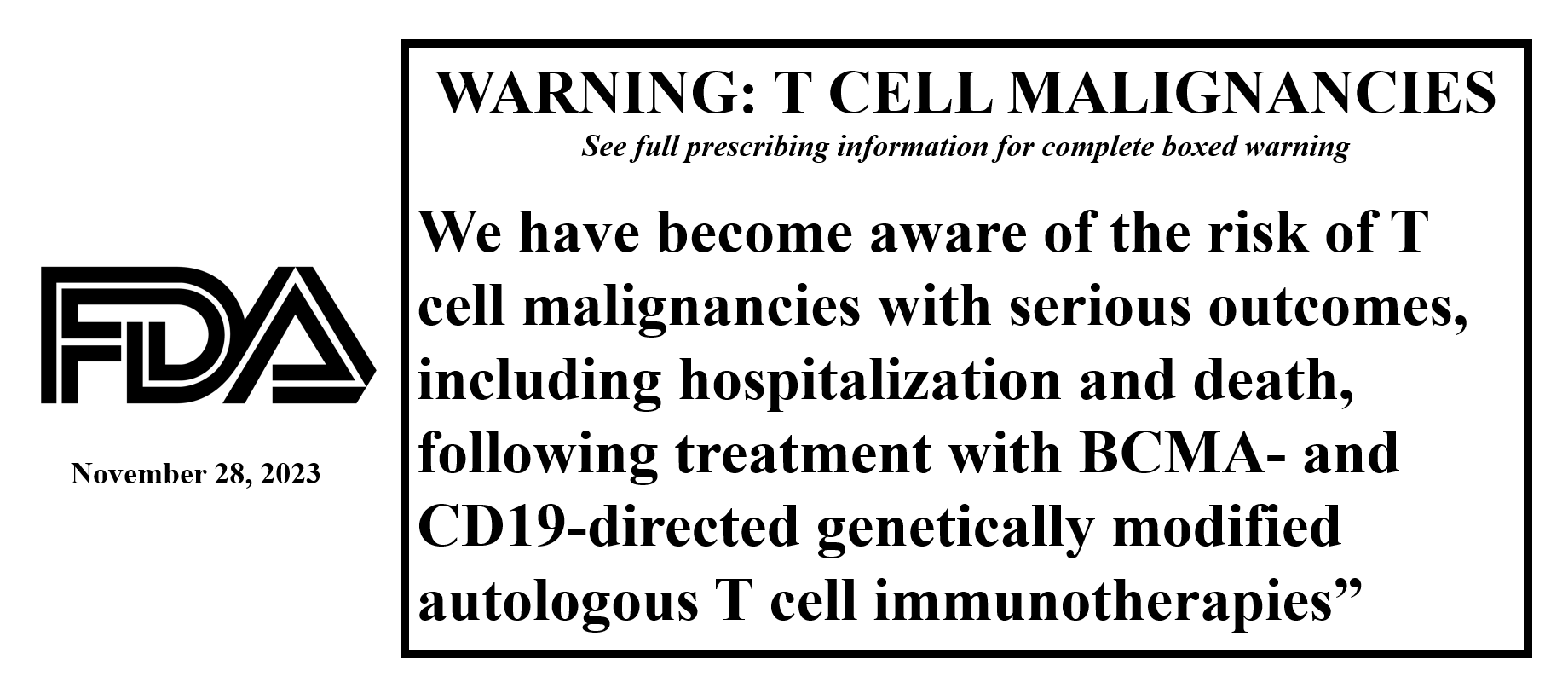
Some doctors reported that a few patients with B-cell cancers treated with FDA-approved DNA-based CAR-T therapy developed lymphoma that contained the CAR-T genetics.
Lymphoma is a cancer of lymphocytes. T-cells are a type of lymphocyte, also called T-lymphocytes. So, if a T-cell becomes cancerous, it is called a T-cell lymphoma. If a CAR-T cell becomes cancerous, it is called a CAR-T lymphoma.
A CAR-T cell lymphoma can occur if a CAR-T cell reproduces uncontrollably and becames cancerous. Fortunately, this rarely occurs. However, each FDA-approved CAR-T therapy had at least one patient develop a CAR-T lymphoma. Therefore, the FDA states it is a “class effect” among CAR-T cell treatments using inserted DNA material.
Since 2017 (the first CAR-T cell product was FDA-approved in 2016), around 30,000 B-cell cancer patients have been treated with DNA CAR-T cell therapies. Therefore, the overall number of CAR-T lymphomas is rare. However, this therapy has been used for only a few years. How many patients could get a CAR-T lymphoma after ten or more years? We do not know.
To put this into perspective, the B-cell cancer patients treated with CAR-T had life-threatening disease, failed multiple standard treatments, and had a high chance of dying without CAR-T treatment. CAR-T cell therapy has induced remission in many patients. Therefore, accepting a rare side effect like this is worth it to most of these patients.
How about CAR-T lymphomas in lupus patients after CAR-T cell therapy?
A big question is for patients with autoimmune diseases, like lupus, most of whom are expected to live long life-spans. Is it worth gambling to possibly develop a CAR-T lymphoma over one’s lifetime, even if the chances are rare?
We do not know if CAR-T lymphoma occurs in autoimmune disease patients treated with CAR-T therapy. So far, no patients with an autoimmune disease have developed CAR-T lymphoma (but the number of treated patients is low). CAR-T cell therapy appears to cause fewer side effects (like complement activation syndrome and severe nerve damage side effects) in lupus patients compared to patients with B-cell cancers. This may have to do with lupus patients having much fewer B-cells killed by CAR-T cell therapy than B-cell cancer patients have.
Lymphoma in DNA vs RNA (and other) CAR-T cell therapies
The CAR-T cell therapies that have caused CAR-T lymphomas have been those where engineered DNA is inserted into the patient’s T-cells (described in detail with the illustrations above). These CAR-T cells multiply (replicate) inside the patient’s body, allowing a rare CAR-T cell to become cancerous, multiply without stopping, and become a cancerous CAR-T lymphoma. CAR-T cell therapies that do not use DNA (like Chimeric autoantigen T-cell receptor (CATCR) T-cell therapies, CAART therapies and RNA CAR-T therapies like Descartes-08) do not reproduce inside the patient’s body and, therefore, should not become cancerous. Autoimmune disease patients may want to consider these forms of CAR-T rather than the DNA-engineered CAR-T if overly concerned about CAR-T lymphoma.
_________________________________________________________________
CAR-T cell therapy side effects seen so far in lupus patients
So far, at least 43 SLE patients have been treated with CAR-T therapy (described below). ICANS was not seen in any of them (as of November 2023 reports). Two of three patients with antisynthetase syndrome (described below) developed mild ICANS that resolved on its own.
CAR-T cell treatments for B-cell cancers also often result in a complete loss of B-cells for a long time. This could increase the risk of some side effects, like infections. CAR-T cell-treated SLE patients also develop a complete loss of circulating B-cells. However, B-cells begin to come back after around 100 days. Most importantly, these appear to be normal B-cells, not the bad ones that previously produced the dangerous lupus autoantibodies (like anti-dsDNA). This is important because normal B-cells are essential for fighting off infections and responding correctly to vaccinations.
CAR-T cell therapy for lupus can cause many other potential side effects. One of the most important, especially in the case of autoimmune disease patients, is that of infections due to chemotherapy and low B-cell levels. Patients treated with two chemotherapy drugs (cyclophosphamide and fludarabine) end up with very low white blood cell counts (white blood cells are necessary for fighting off infections). The CAR-T cells cause a total absence of B-cells for approximately three months in SLE patients. Fortunately, no CAR-T cell-treated SLE patients (43 thus far) have had severe infections.
____________________________________________________
CAR-T cell-treated systemic lupus erythematosus patients (as of April 2024)
———-
The ground breaking case
In 2021, doctors at the University of Erlangen-Nuremberg (led by Dr. Georg Schett) treated a 20-year-old woman with severe systemic lupus erythematosus (SLE) with CAR-T cell therapy. She had kidney, lung, heart, joint, and skin involvement, along with high anti-dsDNA and low C3 and C4 complement levels. She had failed numerous therapies, including hydroxychloroquine, steroids, cyclophosphamide, mycophenolate mofetil (CellCept), tacrolimus, belimumab (Benlysta), and rituximab (Rituxan). The treatment was safe and caused no significant side effects. She went into complete remission, including normalization of her anti-dsDNA and complement levels, by day 44 after CD-19 CAR-T cell infusion. As of June 2024, she was still in remission, more than 2 1/2 years after one CAR-T cell treatment, living an excellent quality of life and taking no lupus medications.
Note: although she is the first lupus patient reported in the medical literature, Chinese doctors treated 2 lupus patients with CAR-T therapy around 1 1/2 years previously. However, their doctors published their results later (described below).
The ground breaking case
This report on the 20-year-old woman was followed by 4 additional patients in 2022 with severe, treatment-resistant SLE who had similar results as the first patient (remission, improved energy, and few side effects). Anti-Smith antibody even resolved, which we do not typically see happen in well-treated SLE patients, even those who go into remission with treatment. Three patients had high levels of interferon-alpha before therapy and, after therapy, had no detectable levels. Interferon-alpha is a cytokine (immune system molecule communicating between cells) that is elevated in most patients with moderate to severe systemic lupus. The resolution of interferon-alpha is remarkable. (As a side note, the FDA-approved lupus drug Saphnelo works by interfering with interferon type 1 and interferon-alpha activity).
In February 2024, Dr. Schett’s team reported the results of all 8 initially treated patients (including an additional 3 patients). It showed that all 8 SLE patients went into and stayed in remission (with no significant side effects). All of them have more than a year since their CAR-T cell therapy. Five patients developed CRS, and three patients needed one dose of tocilizumab (Actemra) for cytokine release syndrome and did well. One patient developed pneumonia, and 3 patients developed new-onset hypogammaglobulinemia (low globulin levels that increase the risk of infection). One patient had pre-existing hypogammaglobulinemia due to previous rituximab therapy. Upper respiratory tract infections (URTI) were common, and 4 patients developed COVID infection. However, COVID and URTIs are common in the general population as well.
No patients developed ICANS.
The first lupus patients treated with CAR-T therapy were in China
In China, 13 patients with severe SLE were treated with BCMA combined with CD-19-directed compound CAR-T cells (discussed below) and were reported in November 2023. Although Dr. Georg Schett’s patients described above were described in the medical literature first, the first patients treated in this Chinese series received CAR-T cell therapy earlier. All thirteen patients went into drug-free remission, and there were no major side effects. All dangerous lupus autoantibodies disappeared. Normal B-cells producing important immune system molecules (immunoglobulins) returned in all patients. All patients have been followed for over a year, and two patients for over 3 ½ years. These two patients were the first in the world treated with CAR-T cell therapy (per published reports to date).
Another 12 Chinese patients treated with BCMA/CD-19 CAR-T
Another Chinese site, Zhejiang University School of Medicine, Hangzhou, China, reported treating 12 patients with severe, treatment-resistant SLE with CAR-T cell therapy directed towards both BCMA and CD-19. This group used both low-dose and higher-dose CAR-T. All patients developed mild, treatable CRS, and none developed ICANS. Several developed infections (2 neocoronavirus, one pneumonia, and one gastrointestinal tract) that resolved with treatment. All patients achieved low-disease activity or remission. The doctors reported these results in a brief report (called an abstract). They did not give all the details, such as which patients went into remission and which did not. However, it is remarkable that all these patients with severe SLE went into at least low disease activity.
Novartis’ YTB323 CAR-T therapy (rapcabtagene autoleucel) and SLE
6 SLE patients were treated with a CAR-T cell therapy called YTB323 (rapcabtagene autoleucel) (discussed below) and reported on in March 2024. Cytokine release syndrome occurred in 4 patients and was treatable and resolved in all four patients. Only 4 of the patients were assessed long enough after CAR-T infusion to assess disease activity. All 4 improved (by the SLEDAI score and physician global assessment), and one patient went into drug-free remission. Five of six patients’ anti-dsDNA levels decreased.
None of their patients developed ICANS. Low gammaglobulin levels were a common side effect. This finding increases the potential for infections. Novartis reported that one patient developed pneumonia but did not require intensive care unit care.
This appears to be a promising CAR-T cell therapy, and I look forward to their future data results.
Kyverna’s KYU-101 used in lupus nephritis
In March 2024, Kyverna reported early results using their KYU-101 CAR-T therapy in two SLE patients with lupus nephritis. They reported results at 28 days after CAR-T infusion for one patient and 90 days for the second patient. Unfortunately, they did not report how their lupus nephritis was doing (i.e., whether proteinuria improved or not). However, anti-dsDNA, C-reactive protein, and complement levels improved in both. One patient had a low platelet count due to SLE (thrombocytopenia) and it resolved after CAR-T therapy.
Pediatric SLE patients treated with CAR-T
A Rome, Italy group treated a 15-year-old SLE patient with severe, treatment-resistant lupus nephritis with CD-19-directed CAR-T cell therapy that was produced using the 2nd generation Prodigy device. Her disease severity went from a SLEDAI score of 12 (severe disease activity) down to 2 (low disease activity). I assume her lupus nephritis may have gone into remission since all the renal domains of the SLEDAI have a score of 4 and higher. Her ANA and anti-dsDNA levels decreased. She did develop grade 2 CRS with fever, rash, and fluid around the lungs (pleural effusion), but these were treatable.
Dr. Georg Schett gave a CAR-T cell therapy lecture at the 2024 European Lupus Society (SLEuro) meeting in Bruges, Belgium, in March 2024. He described a 15-year-old girl with severe lupus nephritis who was treated with a CD-19 CAR-T cell therapy. He showed one slide on the case and stated that the report was “in print” and not formally published yet. She started with very high disease activity (SLEDAI score of 22; anything above 10 is a high disease), and she went into remission after treatment. When treated, she was in complete kidney failure with an estimated glomerular filtration rate (eGFR, a measure of kidney function) of 8 mL/min/1.73m2 (below 10 usually requires dialysis). After treatment, her eGFR improved to 42 mL/min/1.73m2. Her proteinuria decreased from a whopping value of 8660 mg/24 hours of protein down to around 2000. These are truly remarkable results.
At least 43 severe SLE patients treated with CAR-T therapy as of March 2024
So far, at least 43 SLE patients have been treated with CAR-T cell therapy (all described above). Most have gone into drug-free remission. Treatment was overall safe. Most patients developed treatable CRS. No SLE patients have developed ICANs in these reports.
______________________________________________________________________________
Other autoimmune diseases treated with CAR-T
______________
Systemic sclerosis (scleroderma) patients treated with CAR-T therapy
This same group of German doctors used the same CD-19 CAR-T cell therapy in 3 patients with severe systemic sclerosis (scleroderma) and reported their findings in November 2023. All three had profound improvements in some areas (such as the resolution of finger ulcers and improvements in hand function and lung inflammation) and stabilization in other permanently damaged organs. No severe side effects were seen. These were 6-month results.
Myositis patients treated with CAR-T therapy
The same German group also reported the results of CAR-T cell therapy in 3 patients with a severe form of inflammatory myositis called anti-synthetase syndrome (ASS). ASS is usually severe, causing inflammation of the lungs and muscles, and is often accompanied by other problems, like arthritis and rash. It isn’t easy to treat. Two patients were in remission six months and one year after treatment, respectively. The third patient was still awaiting final assessment. All three patients developed cytokine release syndrome (discussed above). Their CRS was successfully treated with tocilizumab in all three. Two patients developed coordination problems (ataxia) due to a nerve complication called ICANS (discussed above). This was mild and resolved in both patients. With all three patients developing some significant side effects, very close monitoring should be done in the future in patients with ASS.
Myasthenia gravis patients treated with BCMA CAR-T therapy
14 patients with myasthenia gravis (an autoimmune disease that attacks muscles) were treated with Descartes-08 (A BCMA CAR-T, described below). After nine months, all had significant improvements. None had major side effects. CRS and ICANS did not occur. Six months after CAR-T therapy, myasthenia gravis antibodies increase, possibly becoming clinically important at the one-year mark. Therefore, therapy may need to be repeated regularly (such as yearly).
Neuromyelitis Optica
Neuromyelitis optica (NMO) is a severe autoimmune disease that attacks nerves of the brain and spinal cord and can be deadly. Doctors gave BCMA-directed CAR-T cell therapy to 12 patients with severe NMO and reported their results in March 2024. After therapy, all patients improved, reported less disability, and had better quality of life. Eleven of the 12 patients had not flared, and all patients’ AQP-4 antibodies decreased. This is significant since the AQP-4 antibody is important in causing inflammation and damage in this disease. Reduced levels are usually associated with better disease control. The median duration of follow-up was only 5.5 months. Therefore, we look forward to longer follow-up reports on these patients in the future.
Some patients with NMO have other autoimmune diseases (like SLE, Sjogren’s, and rheumatoid arthritis). Three of these patients also had Sjogren’s disease, and one had rheumatoid arthritis, and their results are noted below.
Sjogren’s Disease
Sjogren’s disease is an autoimmune disease that attacks the fluid-secreting glands of the body. Dry mouth, dry eyes, dry itchy skin are some of the problems it causes but it also frequently attacks other organs like the nerves, lungs, liver, and kidneys. Three of the NMO patients described above, treated with BCMA CAR-T cells, also had Sjogren’s disease. We do not have final results on one of the patients. However, two patients had impressive results with improvements in dry eyes (improved tear break up time and Schirmer’s tests), dry mouth (improved unstimulated salivary rate testing), and reduced disease activity as measured by ESSDAI. One patient’s anti-SSA was completely resolved while the other’s anti-SSA markedly decreased. It will be interesting to see if it resolves as well over time.
These results suggest that anti-SSA may be produced primarily by the plasma cells of the body rather than by younger B-cells. BCMA may be a better CAR-T target than CD-19 for anti-SSA-positive Sjogren’s disease, if this is true.
Rheumatoid Arthritis
One of the NMO patients reported above, treated with BCMA CAR-T, also had rheumatoid arthritis, an autoimmune disease that causes deformed, crippled joints if not treated. The BCMA-directed CAR-T therapy resulted in remission for the patient.
NOTE: I did not do an exhaustive search for all autoimmune diseases that may have been treated to date with CAR-T therapy. Above are the ones I found. If you know of others, please comment above at “Leave a Comment.)
Could CAR-T Cell therapies be a cure for autoimmune diseases?
So far, there are no cures for autoimmune diseases like SLE. We can treat them, but most patients must take medicines for the rest of their lives (especially SLE patients).
If any of the patients above who are in drug-free remission stay that way for the rest of their lives, then “YES!” those patients can be said to have been cured of their disease by CAR-T cell therapy. Wouldn’t that be wonderful? We must wait and see.
One thing to note is that three SLE patients treated with CD-19 CAR-T reported above were anti-SSA positive. Although the anti-SSA levels decreased, it did not disappear. This tells me that at least in some patients, autoreactive B-cells producing “bad” autoantibodies can persist after CD-19 CAR-T cell therapy for lupus. One must wonder if there could be a chance for recurrence of SLE in that patient in the future.
Interestingly, the BCMA therapy used in the two Sjogren’s patients caused one patient’s anti-SSA to resolve and the other’s to become normal. It was still decreasing when checked last time, so it may also resolve (but we do not know). Since BCMA-directed therapy targets plasma cells rather than younger B-cells, these results suggest that the plasma cells may be most important in producing anti-SSA. BCMA-directed therapies may be best for Sjogren’s disease, but this is only conjecture on my part.
This raises the question of whether some SLE patients are more likely to respond to some CAR-T cell treatments than others. It will become essential to see if this can be figured out beforehand using newer technology, such as machine learning-assisted endotype identification.
CAR-T cell therapy will not improve symptoms due to permanent damage
Many patients with autoimmune diseases like lupus often have permanent organ damage. Around one in three SLE patients will have organ damage a year after their diagnosis, and around half will have organ damage after five years. Some areas of organ damage will result in problems that cannot be reversed by CAR-T therapy. This includes pain from joint, tendon, and muscle damage; vision loss from eye damage; shortness of breath or chest pain if lung or heart damage; and permanent hair loss or skin color changes from skin involvement like discoid lupus. SLE patients and others must realize this before starting CAR-T cell therapy for lupus.
CD-19 CAR-T Cell treated patients still respond to vaccines
A big problem with some B-cell-depleting therapies, such as rituximab, is that they cause a loss of response to vaccines. Some of the sickest COVID-19 patients I saw (including deaths) during the COVID-19 pandemic were rituximab-treated patients who did not respond to the COVID-19 vaccines.
The 8 CAR-T treated SLE patients reported on in 2022-2023 by Dr. George Schett’s group showed that they still had vaccine responses to previous rubella, measles, mumps, Epstein Barr virus, varicella zoster, pneumococcus, and tetanus vaccines. This is important because it means patients do not need to be revaccinated.
________________________________________________________________________________________________________________
Advice for lupus patients after CAR-T cell therapy
Although the CAR-T cell-treated patients have thus far had impressive disease-free results after treatment, lupus patients still have the genetic predisposition for developing problems from the disease again. Scientists have discovered over 180 genes that increase the risk of developing lupus.
For example, although the CAR-T treatment rids the body of bad B-cells that produce dangerous autoantibodies, we are not sure these “bad” B-cells primarily cause these disease. SLE has other types of overactive white blood cells (such as T-cells, dendritic cells, and polymorphonuclear cells).
Other immune system abnormalities exist in SLE patients, such as complement deficiencies and overproduction of some immune system molecules (like type I interferon). If SLE is primarily caused by one of these non-B-cell mechanisms, then that person is at risk for the disease returning. Also, SLE patients probably differ in what mainly drives their disease.
How to possibly prevent lupus from returning after CAR-T therapy
I have some recommendations for SLE patients treated with CAR-T (but please check with your treating rheumatologist; I cannot give formal medical advice without knowing the reader’s entire history, physical exam, labs, and medical history).
Consider taking hydroxychloroquine after CAR-T Cell therapy for lupus
Hydroxychloroquine (HCQ, Plaquenil) is one of the safest drugs to treat SLE. HCQ has numerous benefits in SLE patients, such as reducing the risk of major organ involvement and antiphospholipid antibody production. More importantly, it may reduce the evolution of “at-risk” people in developing SLE, which is pertinent to its use after CAR-T therapy.
In addition, the most significant potential side effect of HCQ, vision loss due to eye damage from retinopathy, should be rare as long as patients get a yearly visual field 10-2 and a yearly spectral domain ocular tomography coherence test. People of Asian descent should get an annual third test (a VF 24-2 or a VF 30-2).
Therefore, I would recommend that patients consider taking hydroxychloroquine and avoid the following lupus triggers after CAR-T therapy.
Avoid situations that can trigger the occurrence of lupus
Most people born with the genes that cause SLE do not develop SLE. Environmental triggers probably play a prominent role in people developing SLE. Therefore, it makes sense to avoid known lupus triggers as much as possible (see the list below):
- Abide by ultraviolet light–protection measures (click the UV light icon at the bottom of the Lupus Secrets page).
- Floss and brush teeth daily to help prevent periodontal disease and tooth decay.
- Ask your doctor to monitor your vitamin D level; take a vitamin D supplement if it is less than 40 ng/mL.
- Never smoke cigarettes; avoid second-hand smoke.
- Avoid sulfa antibiotics (add to allergy list).
- Do not eat alfalfa sprouts or mung bean sprouts.
- Consider healthy use of moderate alcohol drinking when old enough (ask your doctor first).
- Eat a diet rich in omega-3 fatty acids (flaxseed, cold-water fish, chia seed, walnuts, etc.).
- Consider eating “resistant starches” regularly (legumes, peas, overnight oats, etc.).
- Consider decreasing exposure to phthalates (lipstick, plastics, cosmetics).
- Learn to cope with stress; do daily breathing exercises, and practice mindfulness.
- Get at least 7 hours of sleep each night.
- Avoid the herbal supplement Echinacea.
- Avoid exposure to pesticides.
- Avoid using hair dyes.
CAR-T cell therapy may not help some systemic inflammatory diseases
So far, CAR-T cells have been designed to destroy B-cells. Therefore, systemic autoimmune diseases where the B-cells produce dangerous autoantibodies that attack the patient’s tissues are most likely to benefit. Thus far, CAR-T cell therapy has helped patients with some of these diseases, including SLE, systemic sclerosis (also called scleroderma), anti-synthetase syndrome polymyositis, and myasthenia gravis. CAR-T therapy has also helped mice with a multiple sclerosis-like disease.
Hopefully, CAR-T cells will help other autoimmune diseases where B-cells produce autoantibodies. These other diseases that could potentially benefit include rheumatoid arthritis, Sjögren’s disease, antiphospholipid syndrome, Grave’s disease, pemphigus, polyangiitis with granulomatosis, and microscopic polyangiitis (to name a few). Some of these diseases, like Sjögren’s disease and systemic sclerosis, have very few effective therapies today. CAR-T cell therapy could significantly improve their treatment if it proves safe and effective for these diseases.
However, I am concerned with Sjögren’s disease, which can have a high burden (large amounts) of B-cells. This could increase the risk for CRS and ICANS, as discussed above. CAR-T cell therapies showing low CRS and ICANS cases should be considered in Sjögren’s disease clinical trials. Such possible therapies that may not cause more side effects in those with a high B-cell burden include Descartes-08 and CAAR-T Therapy.
Diseases that do not have B-cells producing dangerous autoantibodies are unlikely to be helped with these CAR-T cells. This includes diseases like psoriatic arthritis, Crohn’s disease, ulcerative colitis, and ankylosing spondylitis. However, it is possible to use CAR-T cell technology to target other parts of the immune system. I discuss some possibilities below.
The future of CAR-T Cell therapy for people with autoimmune diseases
SLE patients are now enrolling in CAR-T cell therapy clinical trials (listed at the end of this blog post). CAR-T cell therapy would probably become available for SLE patients with severe, treatment-resistant disease if these prove to reproduce effective, safe results.
But how about patients with milder disease? Around 30% of SLE patients in my clinic go into remission using hydroxychloroquine, UV light protection (like daily use of sunscreen), and vitamin D treatment.
However, the rest require immunosuppressant therapies that carry the potential for more side effects, like infections. This group of patients also has higher medical expenses, especially when you factor in emergency room visits, hospitalizations, twice-yearly eye exams for hydroxychloroquine, and multiple doctor visits. Some patients require very expensive biologic drugs and infusions. All this adds up over the patient’s lifetime. Most SLE patients live long, normal life spans.
Treat mild lupus with CAR-T therapy?
Even though CAR-T cell therapy costs around $400,000 for one patient, this is much less than most SLE patients will need during their lifetime to treat their SLE. A Johns Hopkins (Baltimore, Maryland) study reported 2015 lupus costs of around $20,000 per year for patients with mild disease. Indeed, this would be much higher today in 2023. Even with these figures, one CAR-T cell treatment for a patient with mild SLE would equal the cost of less than 20 years with conventional therapies. The vast majority of 20-year-old patients with mild SLE would be expected to live well past 40 years of age.
The cost of drugs for patients with severe SLE can be as high as $100,000 per year. The cost savings in these patients would be met within just a few years after successful CAR-T cell therapy.
If CAR-T cell therapy were to result in permanent remissions, the overall therapy costs would be well worth it. This is not even considering the hidden costs related to better quality of life, work productivity, time saved by not seeing doctors as often, not missing out on social events due to not feeling well, and the freedom from taking medications all the time.
A big obstacle with using DNA CAR-T cell therapy in people with mild lupus is the possibility of CAR-T lymphoma. Few patients with mild lupus would want to take such a risk. If CAR-T therapies that do not cause CAR-T lymphomas (such as the RNA CAR-T cell treatments like Descartes-08) are proven safe and effective, then they would be a more reasonable option. However, their cost could be prohibitive for mild lupus since these therapies may require repeated infusions and therefore are expensive.
Retreating patients with CAR-T cell therapy
If the autoimmune disease returns after CAR-T therapy, retreatment is possible. There should be frozen (cryopreserved) CAR-T cells saved from the patient’s initial preparation. If the patient had a prolonged remission with few to no major side effects, retreatment with these cryopreserved cells could be considered.
CAR-T cell therapy in doctors’ offices vs hospitals
CAR-T cell therapy is complex. Doctors and nurses must closely monitor treated patients for potentially dangerous side effects (discussed previously). Due to the need for rapid identification and proper treatment of side effects, CAR-T cell therapy for lupus patients is administered in specialized centers where highly-trained specialists are available. This includes rheumatologists, hematologists, nephrologists, pulmonologists, and neurologists.
One CAR-T cell therapy for lupus with the advantage of outpatient treatment (doctor’s offices) that is much more convenient is Descartes-08 by Cartesian. Patients treated with Descartes-08 had no problems with either cytokine release syndrome or neurologic problems like ICANS.
If studies confirm the safety of other CAR-T therapies, it could evolve to being administered in outpatient settings in doctors’ offices by other brands as well.
CAR-T cell therapy research study designs
We always like to have randomized, double-blinded, placebo-controlled trials (RCTs) for drug treatments. This means some patients receive fake treatment (placebo), and neither the doctors nor the patients know which they get (the study drug or the placebo).
A downside to CAR-T therapy research is that some require the destruction of lymphocytes using chemotherapy (cyclophosphamide and fludarabine) before getting CAR-T. You cannot give fake (placebo) treatment to these patients and put them at higher risk for infections. Researchers are currently discussing ways to get around the placebo obstacle so that high-quality studies can be done.
Another improvement that researchers should consider for future CAR-T cell therapy research studies is to improve the identification of cytokine release syndrome (CRS). When patients in a CAR-T cell therapy research study develop fever, this is often attributed to CRS. However, patients in the Descartes-08 CAR-T cell therapy research study who developed fever as a side effect did not have the immunologic features of CRS. Therefore, researchers should define CRS using its known immunologic abnormalities rather than a symptom like fever, which other mechanisms could cause.
CAR-T cells from donors (allogeneic CAR-T cell therapy)
The T-cells used to produce CAR-T cells in this blog post came from the patients (called autologous CAR-T cell therapy). Potential problems with this include the fact that these T-cells may not be healthy. They contain the genes that predispose the person to their autoimmune disease. In addition, they may be damaged by previous treatments with immunosuppressants like mycophenolate, steroids, azathioprine, methotrexate, and cyclophosphamide.
Therefore, research is now looking at possibly producing CAR-T cells from healthy donors (called allogeneic CAR-T cells). Initial studies show that allogeneic CAR-T cells work in treating leukemia patients.
Allogeneic CAR-T cell therapies are recruiting patients for clinical trials. In April 2024 I became aware of Fate Therapeutics’ version of a CD-19 CAR-T called FT819.
Suppose this proves to work for autoimmune disease patients. In that case, some advantages include that the allogeneic CAR-T cells are more likely to have healthier genes and not be exposed to immunosuppressant drugs.
In addition, producing autologous CAR-T cells from the T-cells of a patient (autologous T-cells) is a lengthy, complicated procedure that takes time and could fail at any point in the complex process. CAR-T cells from other individuals (allogeneic) could be stored in a doctor’s office and infused as soon as needed, eliminating long wait times.
Figure out which lupus patients will respond best to different types of CAR-T therapies
One of the studies mentioned below (KYV-101 by Kyverna) will study 24 different immune system cells throughout the study to better understand what happens with the immune system after CAR-T therapy. The FDA awarded fast-track designation to Kyverna’s KYV-101 CAR-T clinical trial.
I hope other companies take similar measures to look deeply at the immune system and patient endotypes to understand better what happens. If some CAR-T therapies work in some SLE patients but not in others, it will be essential to figure out which patients are most likely to respond ahead of time. This sort of research is already being done in some SLE drug trials.
CAR-T cells that target only specific “bad” B-cells (leaving healthy cells alone)
Currently, CAR-T cell therapy attacks and destroys all the B-cells in the treated patient. In SLE patients, the B-cells have recovered after around 100 days. However, they have an extended time before this 100 days when there is an absence of B-cells, placing the patient at risk for infections.
Chimeric autoantigen T-cell receptor (CATCR) T-cell therapy
In November 2023, the Johns Hopkins Division of Rheumatology, Baltimore, Maryland, reported a fascinating study. They developed chimeric T-cells (CATCRs) programmed to destroy B-cells that make a specific autoantibody (an antiphospholipid antibody called beta-2-glycoprotein-1 antibody). This was created using CRISPR technology that edits the genes of T-cells. Instead of destroying all the B-cells in the patient (as what happens with CD-19 CAR-T), this method kills only the B-cells that produce that one antibody. This could be helpful in patients with only one “bad” type of B-cell. In SLE, this may be very difficult since there are usually numerous types of “bad” B-cells making dangerous autoantibodies. Nonetheless, this sounds fascinating and helps paint a brighter future.
Regulatory T-cells (Tregs) engineered with specific T-cell receptors (TCRs)
Abata Therapeutics has designed Tregs with TCRs that recognize the actual damaged tissue (in this case the damaged covering of nerves in myasthenia gravis and the damaged insulin producing cells in diabetes type 1). When the infused Tregs come into contact with the damaged tissues, they release substances (cytokines) that decrease inflammation directly at the area (tissue) involved instead of acting throughout the body. They plan on starting clinical trials for myasthenia gravis in 2024 and for diabetes type I in 2025. Like Descartes-08, chemotherapy will not be used for this treatment.
Chimeric autoantibody receptors (CAARs)
CAARs are T-cells that recognize specific “bad” B-cells (autoreactive B-cells). They are engineered only to identify the autoantibodies on the surface of “bad” B-cells, destroy them, and leave healthy B-cells alone. While CD-19 CAR-T cell therapy is likened to hitting the B-cells with a “sledgehammer,” CAAR T-cells are compared to striking the B-cells “like a razor’s edge.” Cabaletta Bio is testing its CAAR T-cells for the skin autoimmune disease called pemphigus.
CAR-T cells that target cells other than B-cells
Fibroblasts are cells that produce damaging scar tissue in autoimmune diseases like rheumatoid arthritis, Sjögren’s disease, and scleroderma. A team in Oxford, United Kingdom, studied CAR-T cells that targeted “bad” fibroblasts in mice with inflammatory arthritis. It showed positive results. Could targeting fibroblasts be a good option? It does have the potential for fewer infections since it does not involve depleting white blood cells. No human studies have been done yet (as of April 2024).
An American group presented laboratory data on a type of CAR-T cell directed at antigen-presenting cells (APCs). APCs play a central role in the immune system abnormalities in SLE. Some SLE patients specifically have abnormal genetic formation of molecules on APCs that may drive their disease. This could be a viable option for these patients. This is very preliminary, and I suspect animal lupus studies would be the next step.
Other Potential CAR-T targets
CD-19 is the target antigen of the CAR-T cells used in most autoimmune disease patients up to this date. However, it is not clear if this is the most effective target. CD-19 is located in all stages of B-cells, excluding the most mature type called plasma cells. One potential problem with this is that the plasma cells may be in important producer of some autoantibodies (like anti-SSA) and therefore may not thoroughly “reset” the immune system. However, thus far, this target appears to be doing well up to several years after CD-19 CAR-T cell therapy. We need to see what happens in these patients after 5-10 years.
Some patients may possibly do better with CAR-T cells aimed at another target, listed below.
CD-20 and CD-22 CAR-T cells
CD-20 and CD-22 are on all the cells CD-19 is, except they are not on immature plasma cells, called plasmablasts, and on plasma cells (mature plasmablasts). It is unknown if CD-20 or CD-21 would make better CAR-T cell targets. However, CD+ T-cells do appear to play an inflammatory role in autoimmune diseases, and CD-20 targeted CAR-T therapy may potentially play a role in its effects on these T-cells as well as the B-cells. There is one CAR-T cell product called IMPT-514 that targets CD-20 and CD-21. It is described below.
BCMA and CD-38 CAR-T cells
CD-38 and a B-cell membrane antigen called B-cell maturation antigen (BCMA) are also attractive targets. They work very differently than CD-19 since they are located on the most mature B-cells (memory B-cells), plasmablasts, and plasma cells.
If some SLE patients have more plasma cell-driven disease (such as via anti-SSA antibodies), these may be good alternative targets. A potential downside of this therapy is that plasma cells are essential for ongoing antibody production, which it learned to do before CAR-T therapy. Due to this loss of plasma cells, it is possible that patients treated with CD-38 or BCMA CAR-T cells may lose their previous vaccine responses and have to be revaccinated. This is theoretical and needs to be studied.
Cartesian Therapeutics’ Descartes-08: A novel mRNA BCMA CAR-T therapy
Cartesian Therapeutics in Gaithersburg, Maryland, produces a BCMA CAR-T cell therapy called Descartes-08. They plan on starting a clinical trial using Descartes-08 in SLE patients soon. Descartes-08 has already been used successfully in 14 myasthenia gravis patients (discussed previously).
Cartesian plans on not giving chemotherapy before treatment in its SLE clinical trials. A potential advantage of not giving chemotherapy is the possibility of fewer infections. Patients with severe SLE will receive six weekly infusions.
Another potential advantage of Descartes-08 is that it uses mRNA technology instead of DNA. Most CAR-T cell therapies have virus-inserted DNA that produces the antigen receptor. The problem with using DNA is that it causes a large reproduction of many additional CAR-T cells inside the patient. Reproducing numerous CAR-T cells inside the patient leads to activating immune system molecules called cytokines. This is probably a significant reason for the many cases of CRS and ICANS discussed above.
Because of the lack of the above severe side effects, Descartes-08 can be given to patients in their doctor’s office, making it much more convenient than going to a hospital as with other brands (as of January 2024).
A Safer Approach: The Advantages of mRNA Technology
Inserting RNA (also called transfecting RNA) into patients’ T-cells to make CAR-T cells (instead of using lentivirus to insert DNA) is theorized to be safer. Since the CAR-T cells are reproduced in large numbers in the lab instead of in the patient, the immune system activation and extensive cytokine production should not occur as with DNA technology. The Descartes-08 studies in myasthenia gravis had no CRS or ICANS cases.
Over time, the amount of RNA CAR T-cells decreases and eventually disappears. Since they target cells that contain BCMA (plasma cells and plasmablasts), premature and mature B-cells produce more plasmablasts and plasma cells, increasing the risk for the re-emergence of autoantibodies. The Descartes-08 studies show that myasthenia gravis antibodies increase around nine months after Descartes-08 CAR-T cell treatment. The levels of autoantibodies stay below the amount considered significant (and cause increased disease activity) until around the one-year mark.
Potential Risks and Future Considerations
The advantages of Descartes-08 include not needing chemotherapy, outpatient treatments instead of being hospitalized, not causing CAR-T lymphoma, and the total absence of cytokine reactivation syndrome and ICANS (severe nerve damage). This needs to be balanced by the downside of possibly needing ongoing yearly infusions. However, we must wait to see what happens to SLE autoantibodies after Descartes-08 treatment. They may act very differently than myasthenia gravias autoantibodies, which begin to increase around nine months after Descartes-08 CAR-T cell therapy.
Newer CAR-T cell production technologies
Reproducing DNA-based CAR-T cells in large numbers is a long process because researchers must do this before infusing the cells into a patient. However, new technology allows them to replicate the cells in less than 2 days and use them in a dose 25 times lower than other DNA-based CAR-T cells. A CAR-T cell therapy called YTB323 (by Novartis) uses this technology. They are recruiting patients in Australia, Germany, Spain, Switzerland, and France for this study.
Another CAR-T production method called the “NEX-T Process” also uses a shorter production time and allows lower doses of CAR-T. NEX-T Process-produced CAR-T cells may be more effective and have fewer side effects. A clinical trial using CD-19-directed NEX-T CAR-T cells (called CC97540 by Bristol-Myers Squibb and Juno Therapeutics) is recruiting SLE patients at many sites in the United States and Europe. Some are currently recruiting patients. Click on the link to see the contact information.
Current CAR-T Cell Therapy for Lupus Clinical Trials
A search for clinical trials at clinicaltrials.gov (January 2024) shows numerous proposed clinical trials for CAR-T therapy in SLE.
If you are interested in participating in these trials, read over the inclusion and exclusion criteria at the links below. If you do not satisfy these criteria, there are ways of receiving these drugs through compassionate use. Your doctor can contact them at the links if your doctor thinks you would be a good CAR-T cell therapy patient. For any studies that do not list a contact (like the Descartes-08 study), keep checking the link to the study for updates.
SLE CAR-T Cell clinical trials for lupus listed as of December 2023:
Actively recruiting lupus patients for CAR-T cell therapy clinical trials
- An “off-the shelf” allogeneic CD-19 CAR-T product by Fate Therapeutics (called FT819) is recruiting patients at a Nebraska and Minnesota site. Here is more information on FT819, click here.
- YTB323, discussed above, is recruiting SLE patients (including lupus nephritis patients) in Germany, Spain, Switzerland, France, and Australia. If interested in the study, contact Novartis Pharmaceuticals at email@novartis.com, 888-669-6682, or 416-132-1111.
- A CD-19-directed CAR-T cell called CABA-201 (by Cabaletta Bio) was recruiting SLE patients in Boston, Massachusetts. A site at UC Davis Health in Sacramento, California, will be recruiting patients soon. You can contact Cabaletta Bio at 267-759-3100. This study lists Tammy Trotter at tkyotter@ucdavis.edu and 916-734-6944 as the primary contact. The FDA awarded two different fast-track designations for Cabaletta Bio’s CABA-201: one for SLE and one for dermatomyositis.
- Bristol-Myers-Squibb is recruiting SLE patients in the United States and Europe (numerous sites) for its CD-19-directed NEX-T CAR-T cell therapy called CC97540 (discussed above).
- Kyverna Therapeutics is recruiting patients with refractory lupus nephritis for its CD-19-driven CAR-T therapy for lupus called KYV-101. Their research sites are in Denver, Colorado, and Great Neck, New York. Contact Kyverna at ClinicalTrials@kyvernatx.com and 510-925-2484 if interested. Kyverna will use an artificial intelligence platform called the Immune Profiler by Verily in its study. This will track 24 different immune cell types throughout the study, allowing a much deeper dive into the effects of CAR-T therapy.
- Miltenyi Biomedicine is recruiting SLE patients for its CD-19 CAR-T called MB-CART 19.1. In Germany, researchers treated the first five patients with severe SLE and lupus nephritis with MB-CART 19.1. They reported in November 2023 that all patients went into remission with few significant side effects.
- CD-19 CAR-T therapy for lupus nephritis patients who have failed other therapies at Great Neck, New York, and Denver, Colorado
CAR-T cell therapy for lupus clinical trials that will soon be recruiting patients
- CAR-T therapy targeting CD-19 and CD-20 (IMPT-514) at the University of California Los Angeles is actively recruiting patients in San Francisco and Los Angeles. To inquire about the study, click on this clinical trials .gov link.
- Descartes-8 (an mRNA-based CAR-T cell therapy discussed above) is a proposed clinical trial in SLE Cartesian Therapeutics is recruiting patients at a San Diego Site.
- Pharmaceutical company Galapagos (which has both US and European offices) is planning an SLE clinical trial for its CD-19 CAR-T called GLPG5101. Its leukemia studies showed no severe CRS or ICANS cases.
- Autolus Therapeutics plans a clinical trial in SLE for its CD-19 CAR-T called Obe-cel in 2024.
- Sana Biotechnology has applied to the FDA to do a clinical trial in SLE and ANCA-associated vasculitis patients using SC291, allogeneic (meaning they come from donors and not from the patients themselves) CD-19 CAR-T cells.
- iCell Gene Therapeutics is planning a clinical trial in the US using a compound CAR-T (cCAR-T) directed towards CD-19 and BCMA in patients with severe SLE.
- Dr. Georg Schett and his team (the same team that reported on the first published treated SLE patients) will be using MD CAR-T 19.1 in severe SLE patients in Germany.
Chinese CAR-T cell therapy for lupus clinical trials
- Chinese sites were recruiting patients for CD-19-targeted CAR-T cell therapies.
- Numerous others for CD-19 were not recruiting yet. One study in China was recruiting subjects with SLE, Sjogren’s disease, systemic sclerosis, inflammatory myositis, ANCA-positive vasculitis, and antiphospholipid syndrome using allogeneic CAR-T cells (from donors instead of from the patients themselves. The name of this CAR-T cell therapy is Universal CAR-T cells or BRL-301 (Allogeneic Chimeric Antigen Receptor T Cell Injection Targeting CD19 Gene).
- A study in China using CAR-T cells directed at both CD-19 and BCMA was recruiting SLE patients.
- Another Chinese study using BCMA-targeted CAR-T cells was recruiting SLE patients.
CAR-T cell therapy clinical trials for other autoimmune diseases
- Myasthenia gravis patients with Descartes-08 in multiple US sites = they are recruiting patients
- MuSK-positive myasthenia gravis, multiple US sites
- Inflammatory myositis, University of California Irvine (Cabaletta Bio’s CABA-201 for dermatomyositis, polymyositis, anti-synthetase syndrome, and immune-mediated necrotizing myopathy)
- Multiple sclerosis, Palo Alto, California using CD-19 CAR-T cells; and another study by Abata Therapeutics using engineered Tregs with specific TCRs (to begin in 2024)
- Diabetes type I by Abata Therapeutics using engineered Tregs with specific TCRs (to begin in 2025)
- Pemphigus using CAAR T-cells (DSG3-CAART) by Cabaletta Bio
- Chinese sites for Sjogren’s disease
- Chinese sites for many different autoimmune diseases
FINAL THOUGHTS ABOUT CAR-T CELL THERAPY FOR LUPUS
The results so far have been incredibly impressive to me and many others. Critics will point out the low number of patients. We have seen many promising treatments in the past for autoimmune diseases like SLE, where the initial studies looked amazing in small numbers of patients, only to fail when studied formally in large numbers.
However, most patients with severe, treatment-resistant SLE went into complete drug-free remission, not needing any lupus medications for as long as 3 ½ years … that is truly miraculous. No therapy has ever shown such excellent results.
We are entering one of the most exciting chapters in treating these diseases. Wouldn’t it be wonderful if they proved to be this effective and very safe? I imagine a day when I would diagnose someone with SLE and be able to tell them,
“I have a one-shot treatment that can probably get you into remission and has a low risk of major side effects, and could possibly cure you of your SLE.”
However, my dream is premature. What happens after 3 1/2 years in these patients? I will watch the CAR-T story closely and hope it pans out. Stay tuned to “The Lupus Encyclopedia!”
For more in-depth information on research studies in lupus:
Read chapter 37 of The Lupus Encyclopedia, edition 2
Also, look up your symptoms, conditions, and medications in the Index of The Lupus Encyclopedia
If you enjoy the information from The Lupus Encyclopedia, please click the “SUPPORT” button at the top of the page to learn how you can help.
What are your comments and opinions?
If you have lupus, what has your experience been? What do you recommend for other patients?
If you know of any CAR-T cell therapies I missed above, please let me know in the Comments.
Do you have any questions to ask Dr. Thomas?
Please click on “Leave a Comment” above to comment.
Please support “The Lupus Encyclopedia” blog post page
Click on “SUPPORT” at the top of the page to learn how you can support “The Lupus Encyclopedia“
49 Comments
 Deb
Deb Donald Thomas, MDModerator
Donald Thomas, MDModeratorDeb: I could not find any. Good luck, I hope they do plan some CAR-T cell therapy trials in SLE soon.
Donald Thomas, MD
 Myung Sin
Myung SinThank you so very much for such a great blog post. It is so much easier to understand, than other journal papers I read so far! I really enjoyed reading it. I’ve been waiting for treatments for lupus to completely heal me for 31 years. I feel that it is getting closer and closer. So I am very hopeful. . It means a lot to me.
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorThanks so much for your kind comments, Myung! It means a lot as I did put a lot of time into it. It was also complicated for me as a rheumatologist. So, I wanted to put out an article that could help doctors and patients alike, and it sounds like I reached my goal. If I help just one person… it was well worth it
🙂
Donald Thomas, MD
 Geraldine
GeraldineHi Dr Thomas…can I ask what is considered to be serious SLE.
I take Azathioprine 200mg and Hydroxychloriquine 400mg. Could I potentially be a candidate or would I need to be on benlysta Rituximab or equivalent?
Thanks for all the highly informative information you provide Donald Thomas, MDModerator
Donald Thomas, MDModeratorEach study is different. Most go by a severity tool called the SLEDAI. You count whatever manifestations are actually active and add up the numbers
A score of 6 or 8 is commonly used.
Here’s a link to learn how: https://www.lupusencyclopedia.com/how-severe-is-your-lupus/Donald Thomas, MD
 Jamie
JamieGreat, easy to understand article. Thanks! How long will it take before something like this gets to market? 5, 10 or 15 years?
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorIt is actually possible now by compassionate use (but have to jump through FDA hoops to get it.)
I would not be surprised if we see patients getting it in 3-5 years since there are already FDA-approved CAR-T cell therapy products out there.Donald Thomas, MD
 Patience Drake
Patience DrakeWish there were trials for RA. Very good posting. Thank you.
 Debbie Buckley
Debbie BuckleyThank you for this fascinating and easy to understand article – I really appreciate you taking the time to simplify down a complex topic and make it more accessible to those of us with these diseases keen to understand it better, and the treatment options. Much more accessible than anything else I’ve read on CAR-T cell therapy.
I’ve read elsewhere recently about successful use of daratumumab (anti-CD38) to treat some cases of refractory autoimmune diseases, including lupus and others. Interestingly, one haematologist I spoke to said they felt that daratumumab was safer than rituximab with respect to covid infections in particular in their patient group (myeloma patients). I found this comment fascinating, especially given that covid is still causing problems for those of us on rituximab, even despite multiple vaccines, etc. Her explanation was that daratumumab had a narrower impact on the immune system compared to rituximab which impacts more broadly, and that it was still possible to produce antibodies from other memory b cells even after plasma cell depletion using daratumumab. Hearing this made me wonder why daratumumab is not being used more broadly in SLE and other B-cell autoimmune diseases, given the ongoing issues with covid infection and rituximab.
If you get a chance I would be fascinated to hear your thoughts on the use of daratumumab to reduce autoantibody producing plasma cells.
Thank you as always for trying to improve our lives and providing a great educational but practical resource.
Best wishes from Ireland,
Debbie Donald Thomas, MDModerator
Donald Thomas, MDModeratorDebbie: Thanks for the kind words. I put a lot of work into the article in the hopes that it would help others understand, so this makes it all worth-while.
I agree that daratumumab is a worthwhile drug to evaluate and it does have potential advantages as you describe.
It has shown benefit in some case studies.
In Germany there is supposed to be a phase 2 clinical trial called the DARALUP trial going on, and for lupus nephritis, and currently, the Mayo Clinic is enrolling patients with lupus nephritis in a daratumumab phase 2 clinical trial that is scheduled to finish later this year.
Fingers crossed!btw, the Descartes-08 CAR-T targets similar B cells as CD-38 and is starting to recruit SLE patients.
Donald Thomas, MD
 Ryan
RyanLoved the article! How does the approval process / timeline work for CAR-T therapies?
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorRyan: Not so sure. The FDA black box warning for CAR-T lymphomas throws an unknown wrench into the answer for this question. I suspect CAR-Ts that would not cause cancer (RNA-based, CAART, CAPCR etc) would have an easier road if proven safe and affective. Wishful thinking would be “could we see an FDA approval in 5 years?”
Currently doctors can apply for compassionate use for an SLE patient with severe disease who has failed everything.
Donald Thomas, MD
 Ryan
RyanSounds promising. Do you know if the Descartes-08 trial is only for those with severe lupus, or do they want mild cases as well? Same question for if the med is eventually approved
 ARW
ARWI have autoimmune-mediated small fiber autonomic and sensory neuropathy (positive TS-HDS antibodies) in addition to SLE. Do you think that nervous system involvement would prevent me from participating in a clinical trial?
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorARW: It is probably OK. I know the main CAR-T studies excluded CNS lupus, but I don’t think it excluded these. It will vary by study.
For example, the Descartes-08 study (an RNA CAR-T) did not exclude neurologic disease at all for the myasthenia gravis study as severe nerve problems have not been noted with it.
As per:Supplement to: Granit V, Benatar M, Kurtoglu M, et al. Safety and clinical activity
of autologous RNA chimeric antigen receptor T-cell therapy in myasthenia gravis
(MG-001): a prospective, multicentre, open-label, non-randomised phase 1b/2a study.
Lancet Neurol 2023; 22: 578–90.Donald Thomas, MD
 Charlie
CharlieHi I was wondering if the patients in the trial experienced complete remission of all their lupus symptoms with their SLEDAI score of 0. Did their “type 2” symptoms (diffuse pain, fatigue, lupus fog, etc.) go away as well?
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorCharlie: That will be very interesting to see. I suspect it will in some patients due to either the “type 2” symptoms being incorrectly labeled as type 2 and their fatigue, aches, and brain fog were actually related to their systemic lupus inflammation; or, they could resolve if lupus inflammation is a driver of central pain amplification. I sure hope it does. We will have to wait and see and hope.
Donald Thomas, MD
 Charlie
CharlieHi Dr. Thomas, I stumbled across this Chinese trial: https://ashpublications.org/blood/article/142/Supplement%201/4835/504325/CD19-BCMA-CAR-T-Cell-Therapy-for-Refractory
Is this the same one as the 13 patient study you described in the article? It looks like it’s different. I’d love to hear your thoughts if it’s a new result.
Thanks,
Charlie Sean Liu
Sean LiuHi Dr. Thomas,
Thank you for this valuable article. My 3.5 YO daughter has recently been diagnosed with pJIA with early lupus. Notably, her disease has an unusual feature of very significant B cell activation. She has very high RF (~500), ACPA (~200), along with ANA+, anti-ds DNA+, SSA+, SSB+. While we had her on Humira+MTX+HCQ at the moment, we were so pessimistic about her future disease progression. Your article on CAR-T is truly our hope. I plan to try everything we can to slow her disease progression until she is 18 years old. Then hopefully if her lupus keeps progressing, CAR-T could be an option for her. I feel that given her B cell activation, even her arthritis could be significantly alleviated by CAR-T.
I also work in a pharmaceutical company working on MS. I was aware of the success of the CAR-T on SLE but did not see all these details or realized the long list of the numerous start-ups that are rushing towards this direction. You did an amazing job in explaining this.
Best,
Sean Liu Donald Thomas, MDModerator
Donald Thomas, MDModeratorSean: so sorry to hear about your daughter.
If she has not been tried on belimumab yet, ask her doc. We try to stay away from Humira for SLE. However, belimumab (Benlysta) works directly on B-cells and especially helps patients who are anti-dsDNA positive and it is FDA-approved for children. Though it is FDA-approved for 5 years old and older (only because 3.5 yo children were not included in the clinical trials) … pediatricians are experts on getting off-label treatments for 3.5 year olds since there are no FDA-approved drugs for such young patients.The primary disease modifying agents for lupus are: hydroxychloroquine, belimumab (Benlysta), and anifrolumab (Saphnelo):
Here is some information about Benlysta:
https://www.lupusencyclopedia.com/saphnelo-vs-benlysta/
https://www.lupusencyclopedia.com/how-benlysta-works-for-lupus-patients/I sure hope she does well,
Donald Thomas, MD
 Sean Liu
Sean LiuThank you Dr. Thomas. We will surely pass the message to her care team. I think they started her on Humira because 1) her main presentation was arthritis; 2) her anti-dsDNA titer just appeared to be weakly positive right before starting steroids/Humira; 3) she did not have other SLE symptoms and her arthritis was well managed under the current combo of meds. If you have further recommendations/questions related to her diagnosis, please feel free to email me. We really appreciate your suggestions.
Back to CAR T, do you think seropositive RA patients may benefit more from CAR T than seronegative ones?
CAR T is already approved for treating children with certain types of leukemia. If/once CAR T is approved for SLE in adults, how soon do you think it can be applied to pediatric lupus patients, like 5-7 years after? Sometimes I just worry that we cannot hold her disease progression for 15 years. It seems so long.
Best,
Sean Donald Thomas, MDModerator
Donald Thomas, MDModeratorThe RA question is interesting. A BCMA CAR-T did get a SPRA patient into remission (the patient had NMO and RA overlap). Since CAR-T cells can be engineered to go after many different types of cells, if researchers discover the primary target to go after in SNRA, then it may work (but I don’t follow the SNRA literature as closely).
A peds lupus clinical trial for CAR-T was just launched. Not sure how long it would take to be clinically available.
Please don’t pass on my comments to the healthcare team to be applied to her case specifically. I can only may general comments based upon very limited information. It is so important to have all information (history, physical exam, test results, failed therapies, etc). However, you can use them in general terms.
 Karl
KarlThanks Dr. Thomas for keeping us updated.
The current data for B-cell mediated autoimmune diseases and CAR-T therapy indeed looks very good. Of course the important caviat is that the data is only short-term data and whether things really turn out to be too different from RTX remains to be seen. I certainly hope that all these results will mean that researchers will focus on B-cell targeting much more, like they should have in the past decades.
I find it quite shocking that trials for conditions such as RA haven’t been launched yet, considering it was one of the first diseases treated with RTX and has a well-established aetiology.
Something that slightly worries me is that I know of several CAR-T trials that should have somewhat long-term follow-up data but never reported any results to begin with (for example the following studies seem to never have reported any results https://www.clinicaltrials.gov/study/NCT05085431?term=Autoimmune%20Diseases&intr=CAR-T&rank=7, https://www.clinicaltrials.gov/study/NCT05030779?term=Autoimmune%20Diseases&intr=CAR-T&rank=9), whilst DSG3-CAART for pemphigus vugaris failed whilst CAR-T aimed at B-cells for antigens of MusK also seems to have had negative results. I hope that reporting of positive results isn’t shifting these results.
Is was also wondering what you are thinking about possible combination treatments such as CAR-T+Efgartigimod to possibly break down some positive feedback loops. Some of the Efgartigimod data looks rather good.
Kind regards,
Karl Donald Thomas, MDModerator
Donald Thomas, MDModeratorKarl: thanks for the in depth comment.
I suspect RA is not one of the first diseases studied because it made sense to begin CAR-T in patients with AI diseases that were immediately threatening the lives of the patients (like SLE, scleroderma, NMO, anti-synthetase myositis). However, if safety continues to be demonstrated, it makes complete sense it could be soon for the RA sphere since 20-30% of RA patients are “treatment-resistant.”
Those particular studies are Chinese based and it looks like they may still be recruiting. I suspect they also may not keep clinicaltrials.gov up dated.
The MusK MG using RNA-based Descartes-08 did have positive results. It just looks like it may need to be given repeatedly (?yearly?)
Unsure about efgartigimod since clinical trials are so early for SLE and Sjogren’s. It sure looks impressive for MG.
I think the next decade will have an explosion of therapeutic breakthroughs (thank goodness)
DT
 Ryan
Ryan Donald Thomas, MDModerator
Donald Thomas, MDModeratorRyan: Thank you! I did not see this one and will add them to the list. I greatly appreciate your finding this abstract.
Dr T
 Ryan
Ryan Vicki
VickiiPSC-derived CD19 CAR T cells in lupus. Actively enrolling, less hospitalization, no leukapheresis. Exciting times!
https://www.clinicaltrials.gov/study/NCT06308978?cond=lupus&term=Ft819&rank=1
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorVicki… and it is “off the shelf”… wow! I’ll spread the news
Thanks!
Donald Thomas, MD
 John Bergman
John BergmanDr Thomas, do you have any thoughts on weather or not Car T cell therapy will be effective for folks with MCTD or overlap syndromes?
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorJohn: I suspect it would. Dr. Georg Schett showed that patients with severe myositis, lupus, and scleroderma responded. MCTD is similar to having overlapping features of myositis, scleroderma, and rheumatoid arthritis.
Exciting prospects
 Petra
PetraI made a Facebook group for CAR-T autoimmune patients, we have plenty of members by now who have done CAR-T and some are just in the process of doing it.
Please join!
https://www.facebook.com/groups/cartautoimmune Sean Liu
Sean LiuI think if you relapse from one CAR T, re-treatment of the same CAR T will likely fail because previous CAR T in the body generates memory T cells that can attack your CAR T. You will need a CAR T for a different target, or a CAR T against same target but using a different antigen binding region. Currently many CD19 CAR T uses murine anti-human CD19. Will have to see if fully humanized anti-human CD19 will generate the same anti-CAR T T cells. This scenario will be similar to the anti-drug antibodies in RA patients. If you fail an anti-TNF inhibitor, say Humira, you can switch to anti-IL-6 inhibitor, such as Actemra, or another anti-TNF inhibitor, such as Enbrel. But it is likely that the biologics against a different target will last longer. I suppose we will have a few different anti-CD19 CAR T in the future, and a few anti-BCMA ones, and a few anti-CD20 ones as well as anti-CD38 ones, hopefully. Then possibly in some patients that keep having relapses, each CAR T can buy 5-7 years, which are still very significant.
One thing we have to see is whether mRNA CAR T can keep working by repeated dosing. In theory once you have anti-CAR T T cells, it will no longer work. But if it does, even not 100%, that will still be quite helpful. My worry is that you need more and more frequent dosing because your anti-CAR T T cells become more and more efficient in clearing them out.
Anyway, those are all my conjectures. Let’s see how things work out.
 Hu
HuThis is a great article. Could you give us some information about CAR-NK treatment of autoimmune diseases?
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorThanks for the kind words. I find this new chapter in SLE to be fascinating and very promising.
Is CAR-NK safer and more effective in SLE than CAR-T? We do not know. We still need actual data. Then we must wait and see what happens long term. Proponents propose theoretical advantages of CAR-NK. However, SLE likes to buck theories.
I look forward to study results (I see there is an active study in China at the moment)
Donald Thomas, MD
 Anne Marie Kirlin
Anne Marie KirlinHi Dr. Thomas
Johns Hopkins now has a Car-T research study going on. I have had a hard time finding details about it but I know they have started it and they are recruiting
Thanks for all the wonderful information you provide to us.
Anne Marie Anne
AnneThe Phase 1 trial at Johns Hopkins is Sana Biotech GLEAM study SC291-102
A hypoimmune allogenic CD19 directed Car-T cell therapy. Anne
Anne
I am dying from cerebral-systemic lupus that is very severe and I am in pain all the time. Can I please be a volunteer for your trial? I am willing to go to your place and bear any risks being in your trial, even if it means the side effects will kill me. I know I will die because of my severe lupus in due time if I do not get car t treatment. I have tried all sorts of treatment, including chemo, ivig, plasmapheresis, and nothing wotks to relieve my lupus. I await to hear from you. Thank you. Kind regard, Astrid Meilasari-Sugiana. astrid.sugiana@bakrie.ac.id
 Clara Long
Clara LongGreta article! Encouraged by this treatment. I have Sjogren’s and like you said, very few effective treatments for my disease. Frustrating to see so much being done toward lupus when quite a few treatments already exist for that. Not suggesting that lupus research stops, but rather, that more Sjogren’s research is pursued. It is much more than dry eyes and mouth (as you stated).
Any idea of clinical trials for Sjogren’s and Cart-T near Tampa, FL?
Thanks a lot!
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorI agree, Clara!
Everytime I get the chance, I stand up at meetings or discuss with those “in power” and say, “we need CAR-T studies for Sjogren’s! It is a B-cell driven disease and the most common systemic autoimmune disease!”Donald Thomas, MD
 Nivia
NiviaHi,Dr. Thomas!
Thank you for such a great and clear information!
I have a question:Do you know if CAR T cell eliminates antiphospholipid syndrome or antibodies? Could kids under 18 years old be treated with this type of therapy? Feeling hopeless and devastated! Thank you, again! Donald Thomas, MDModerator
Donald Thomas, MDModeratorNivia: The quick answer is that no current CAR-T therapies have been proven to help APS (antiphospholipid syndrome).
However, there is hope.
Interestingly, the study from Johns Hopkins directly affected only B-cells that made a particular antiphospholipid antibody (leaving other B-cells alone). While this would not be helpful in a disease that makes lots of autoantibodies (like SLE), it could potentially help diseases where only 1 or two types of B-cells that produce 1-2 autoantibodies (like APS). Below is my excerpt from my article addressing this:
Chimeric autoantigen T-cell receptor (CATCR) T-cell therapy
In November 2023, the Johns Hopkins Division of Rheumatology, Baltimore, Maryland, reported a fascinating study. They developed chimeric T-cells (CATCRs) programmed to destroy B-cells that make a specific autoantibody (an antiphospholipid antibody called beta-2-glycoprotein-1 antibody). This was created using CRISPR technology that edits the genes of T-cells. Instead of destroying all the B-cells in the patient (as what happens with CD-19 CAR-T), this method kills only the B-cells that produce that one antibody. This could be helpful in patients with only one “bad” type of B-cell. In SLE, this may be very difficult since there are usually numerous types of “bad” B-cells making dangerous autoantibodies.
Though scientific breakthroughs often take over a decade to get into practical use, there were at least 30 abstracts/studies/presentations about CAR-T at our recent annual ACR meeting. It appears that CAR-T research is truly exploding (in a good way). With the FDA fast tracking some of these… wouldn’t it be wonderful if we were able to use these treatments sooner than later.
Donald Thomas, MD
[…] But these stunning results—remission in every patient—have fueled a new wave of optimism. More than 40 people with lupus worldwide have now undergone CAR-T-cell therapy, and most have gone into drug-free […]
 Penelope Pestronk
Penelope PestronkWow what a complete explanation. Thank you. It’s very heartening to see these developments and think there is hope for an SLE cure someday. I don’t know how I missed this when it first came out (I see comments back from July) since I subscribe. But I am glad to see it now.
Could you do a piece on the usefulness of colchicine as a treatment for SLE patients?
 Sean Liu
Sean LiuHi Dr. Thomas,
Check this out- new CAR T incorporated anti-TNF and anti-IL6 so CRS is silenced completely. This is still autologous CAR T. I can imagine that if they make this into allogeneic- that will be CAR T with little CRS/ICANS. Very exciting.
https://www.nature.com/articles/s41422-024-01068-2Sean Liu
 Squidmom
SquidmomHi Dr. Thomas, thank you so much for taking the time to put this together. If you ever hear of a trial that would be relevant for Relapsing Polychondritis (autoimmune, very rare), I’d be very interested on finding out more about it. Thank you!
 Donald Thomas, MDModerator
Donald Thomas, MDModeratorSquidmom: I will keep my eyes open
Donald Thomas, MD





Leave a comment